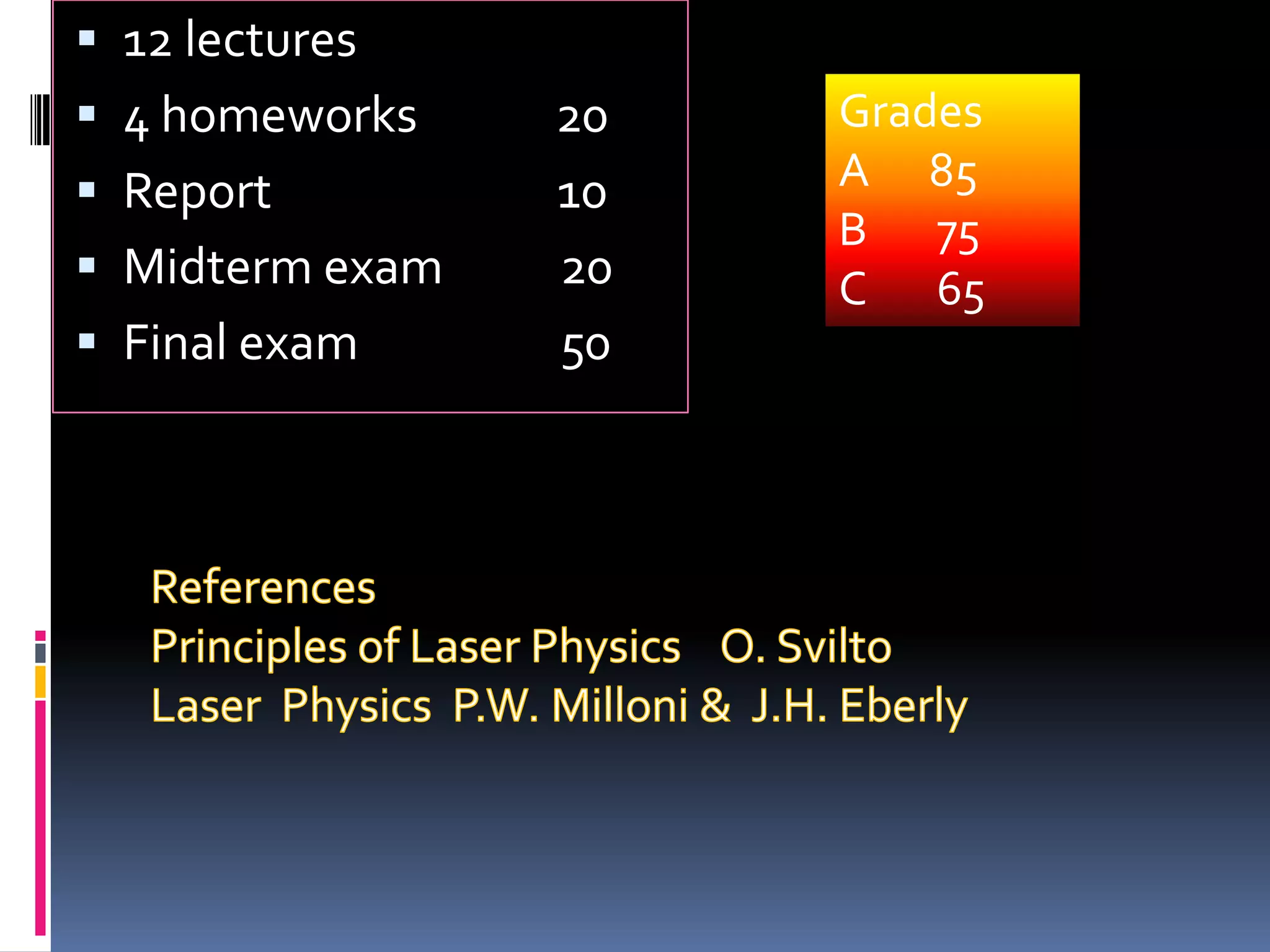
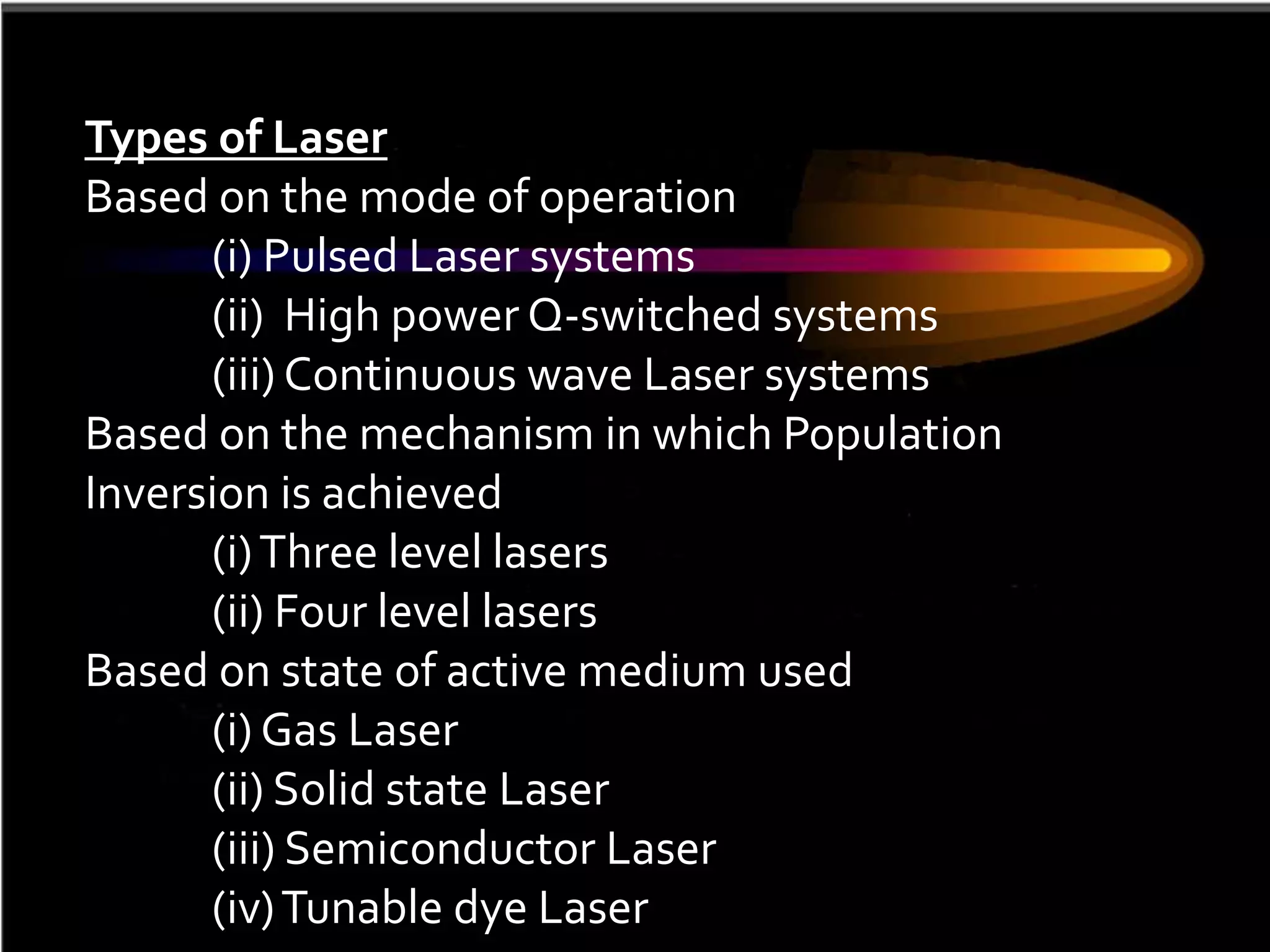
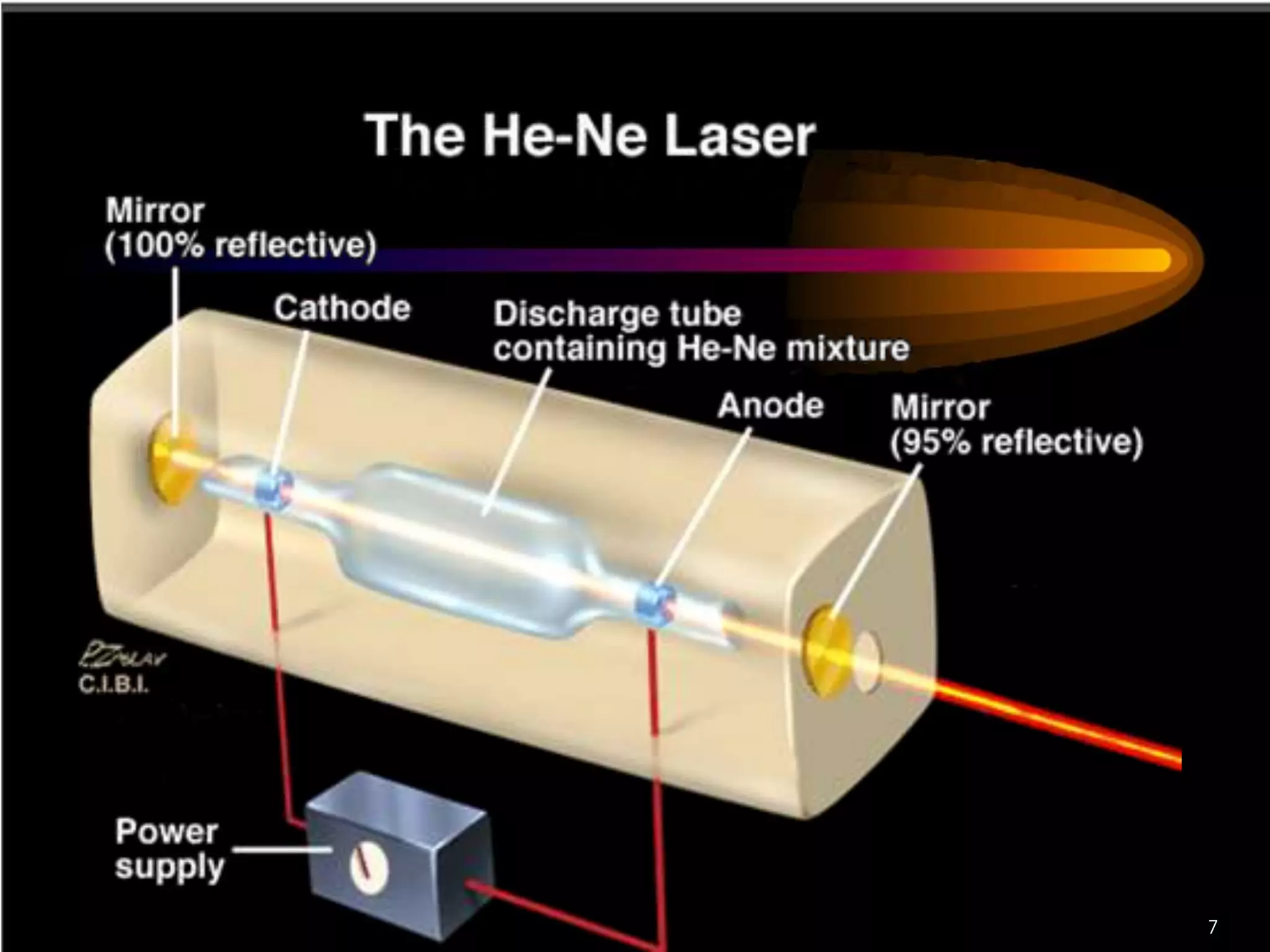
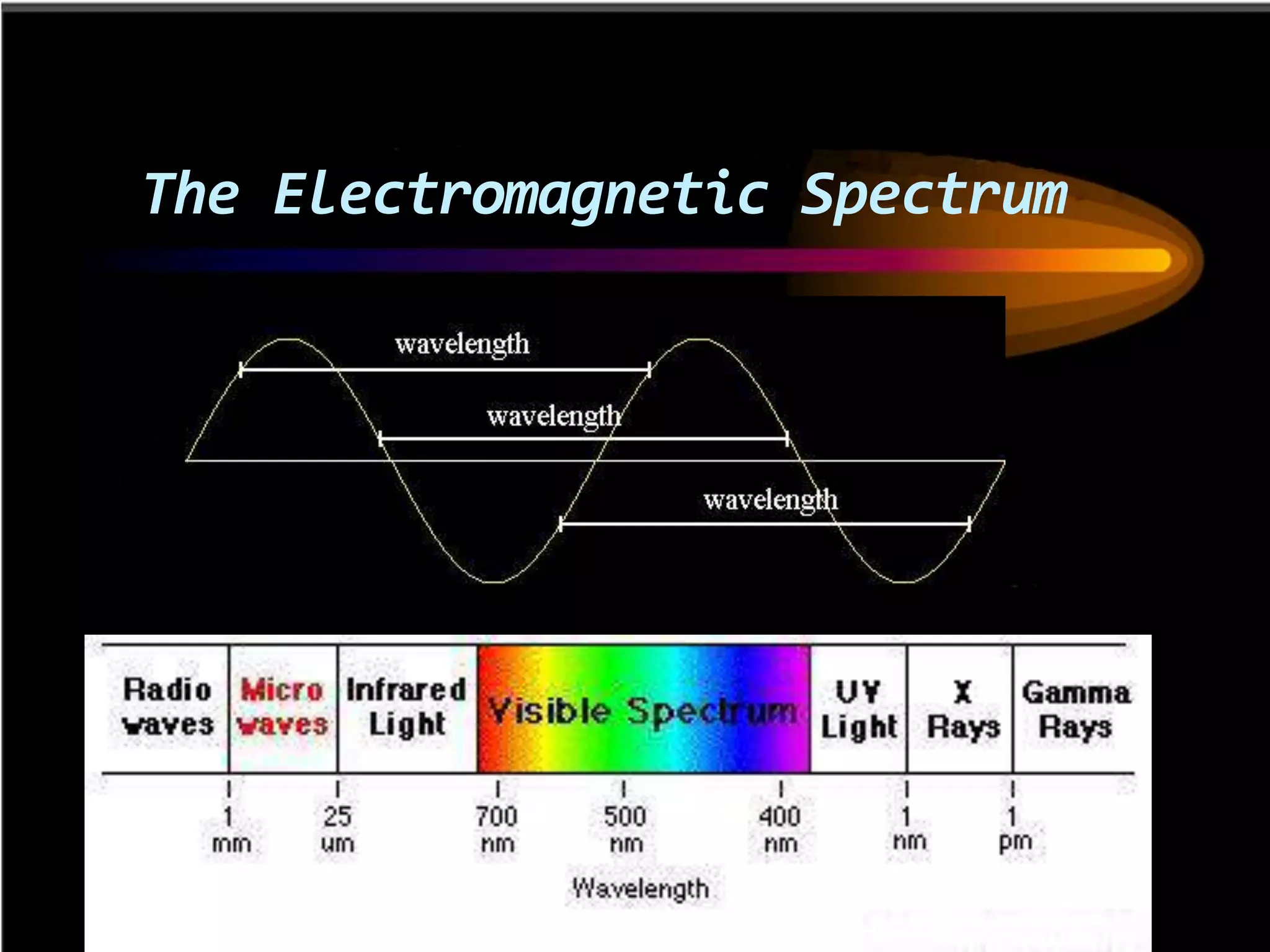
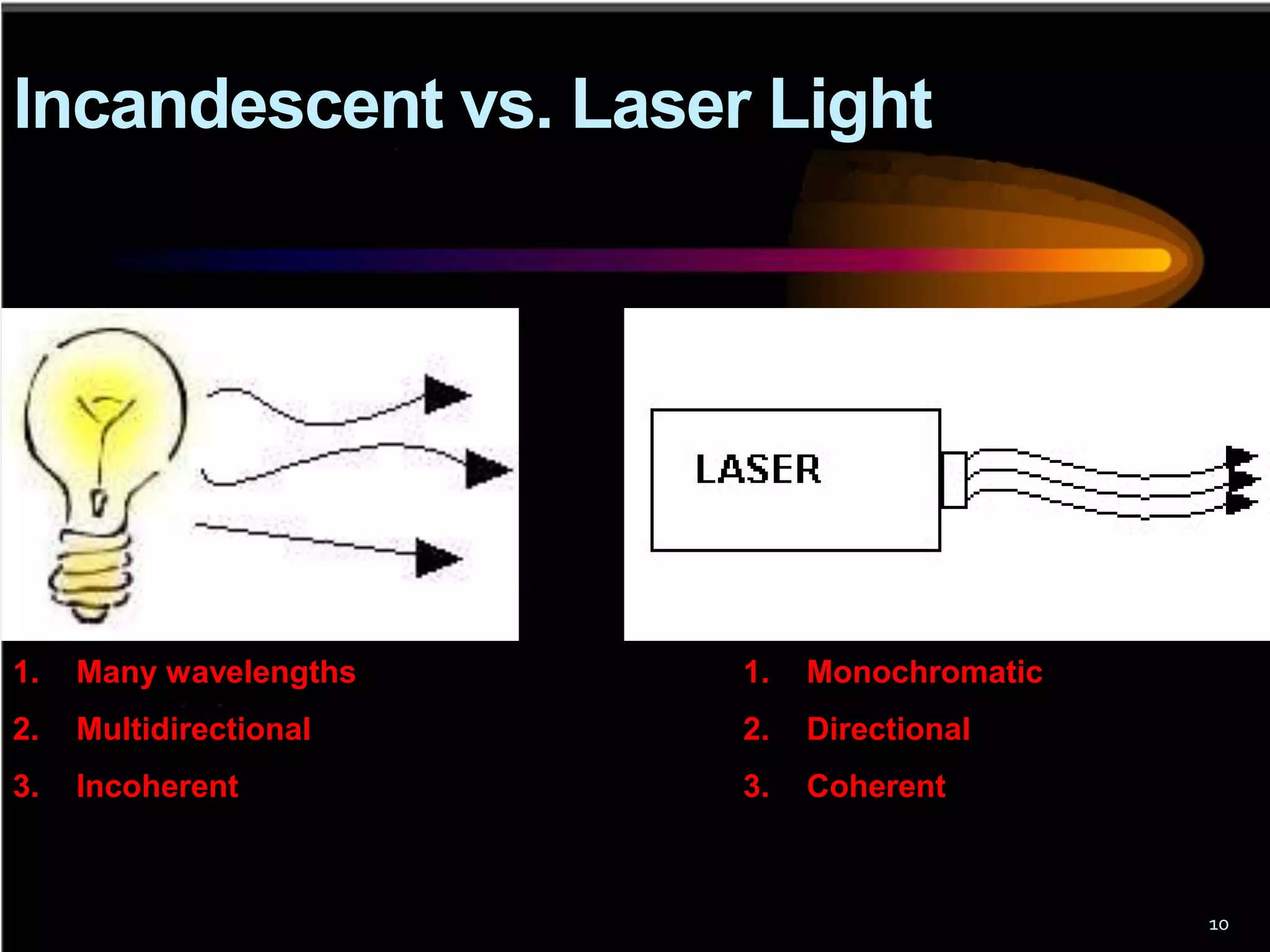
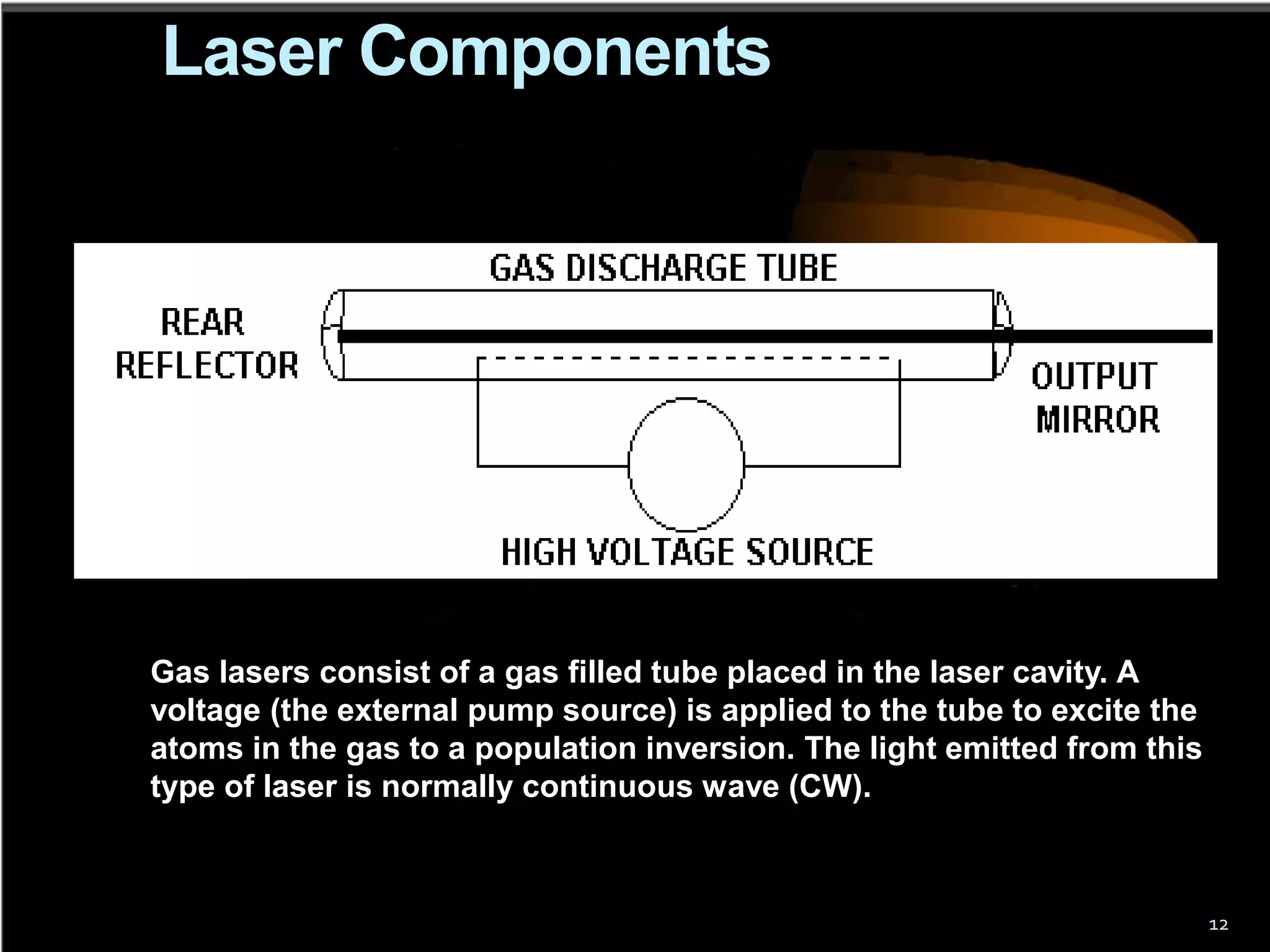
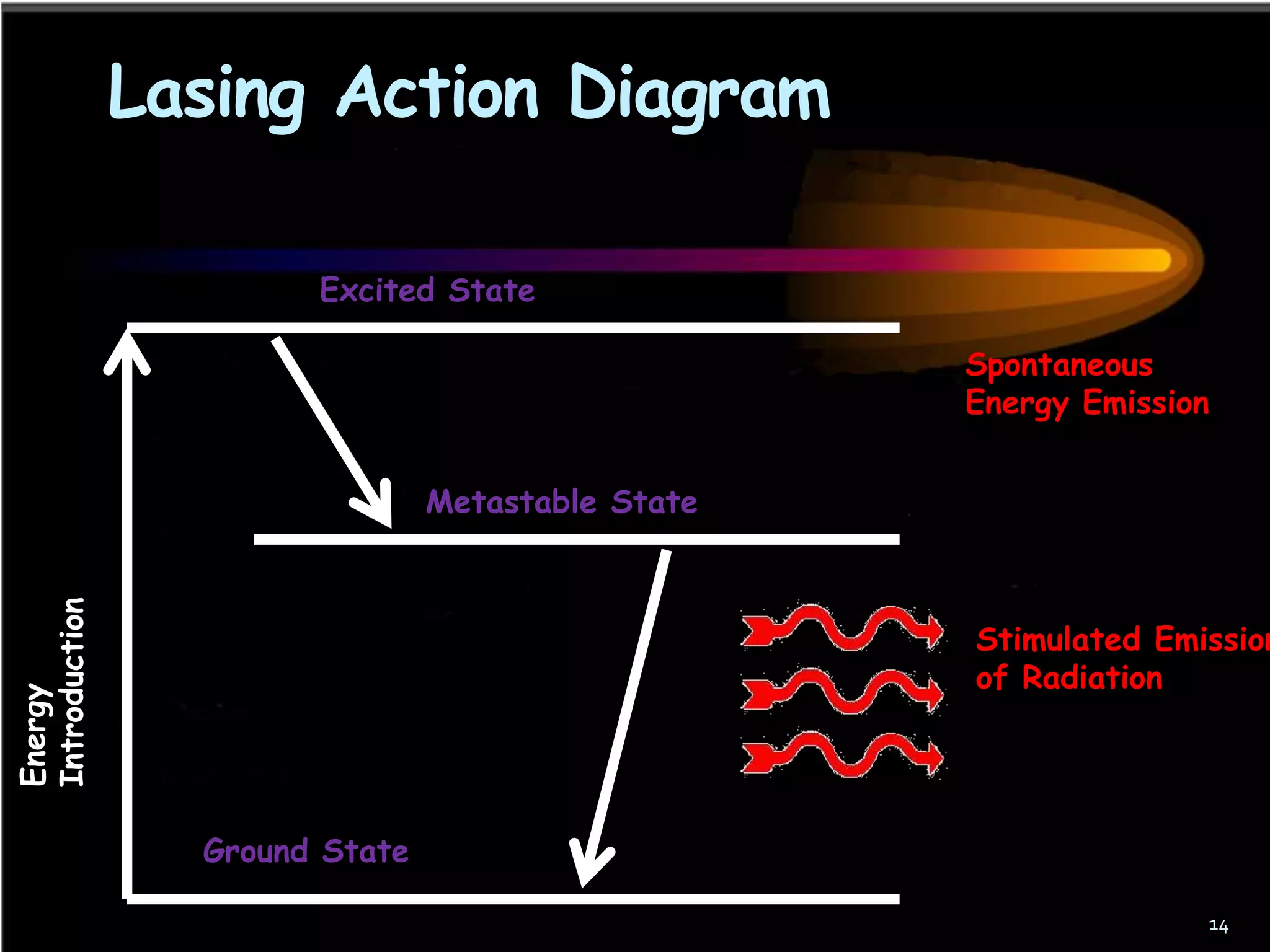
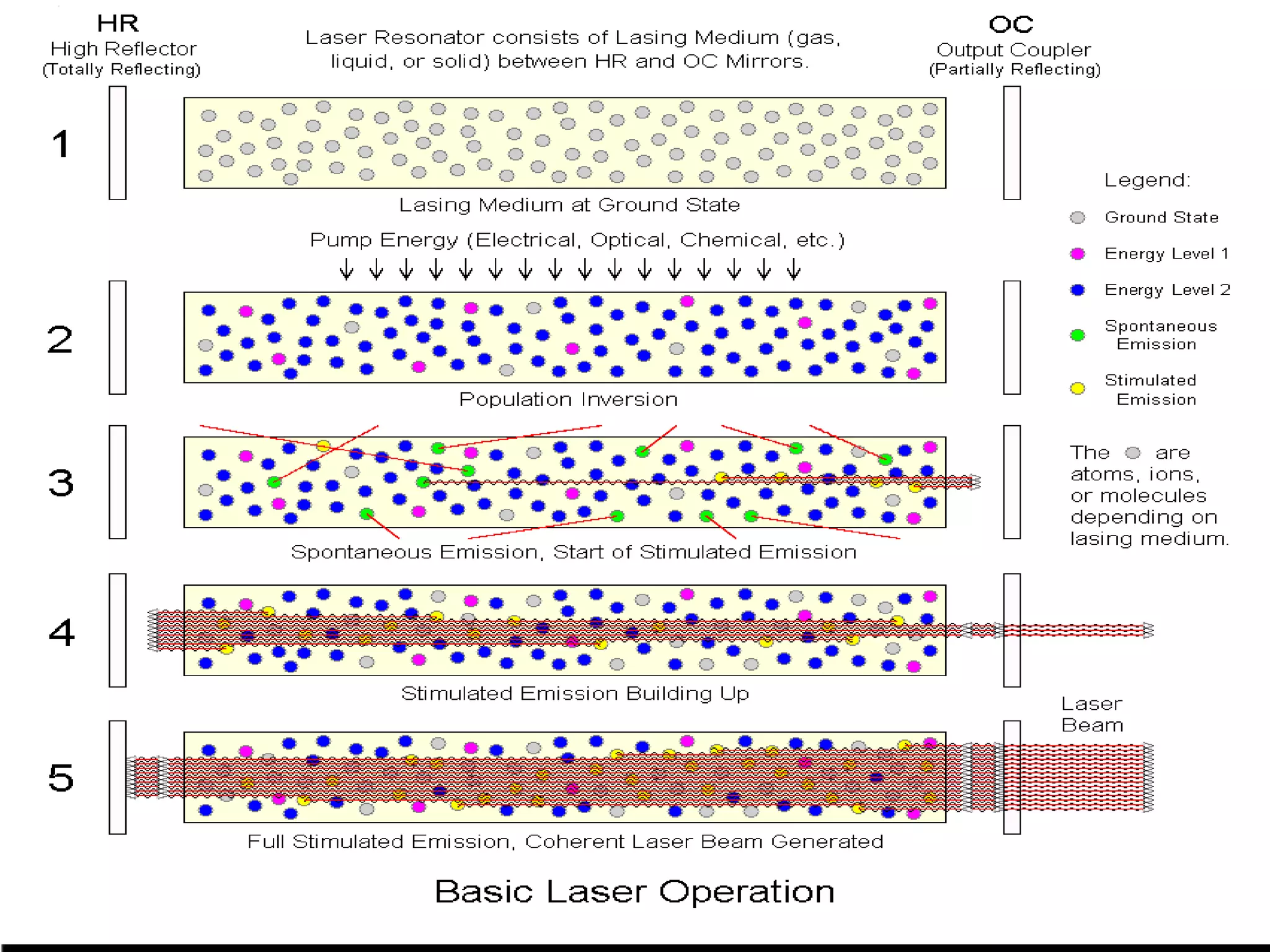
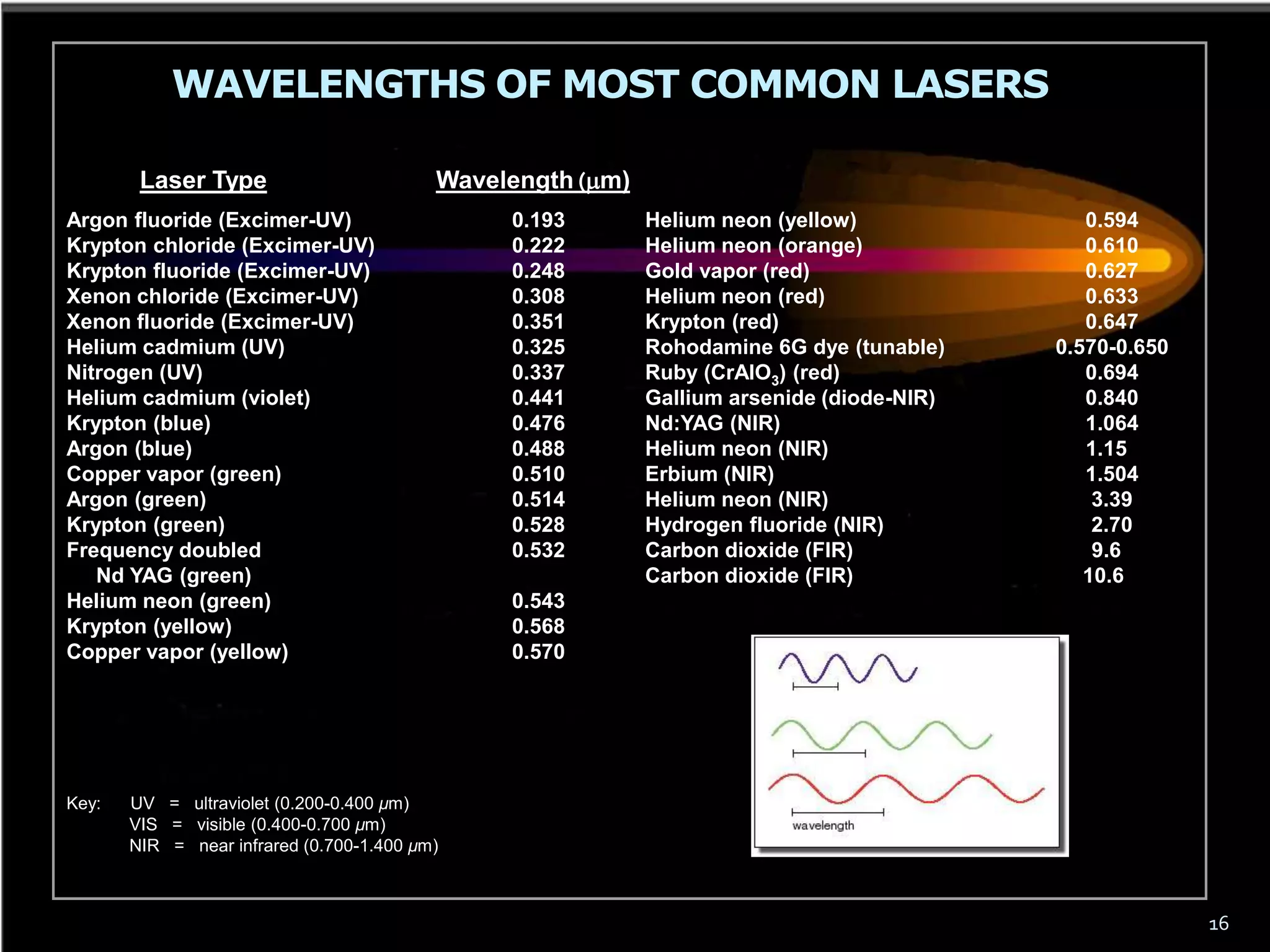
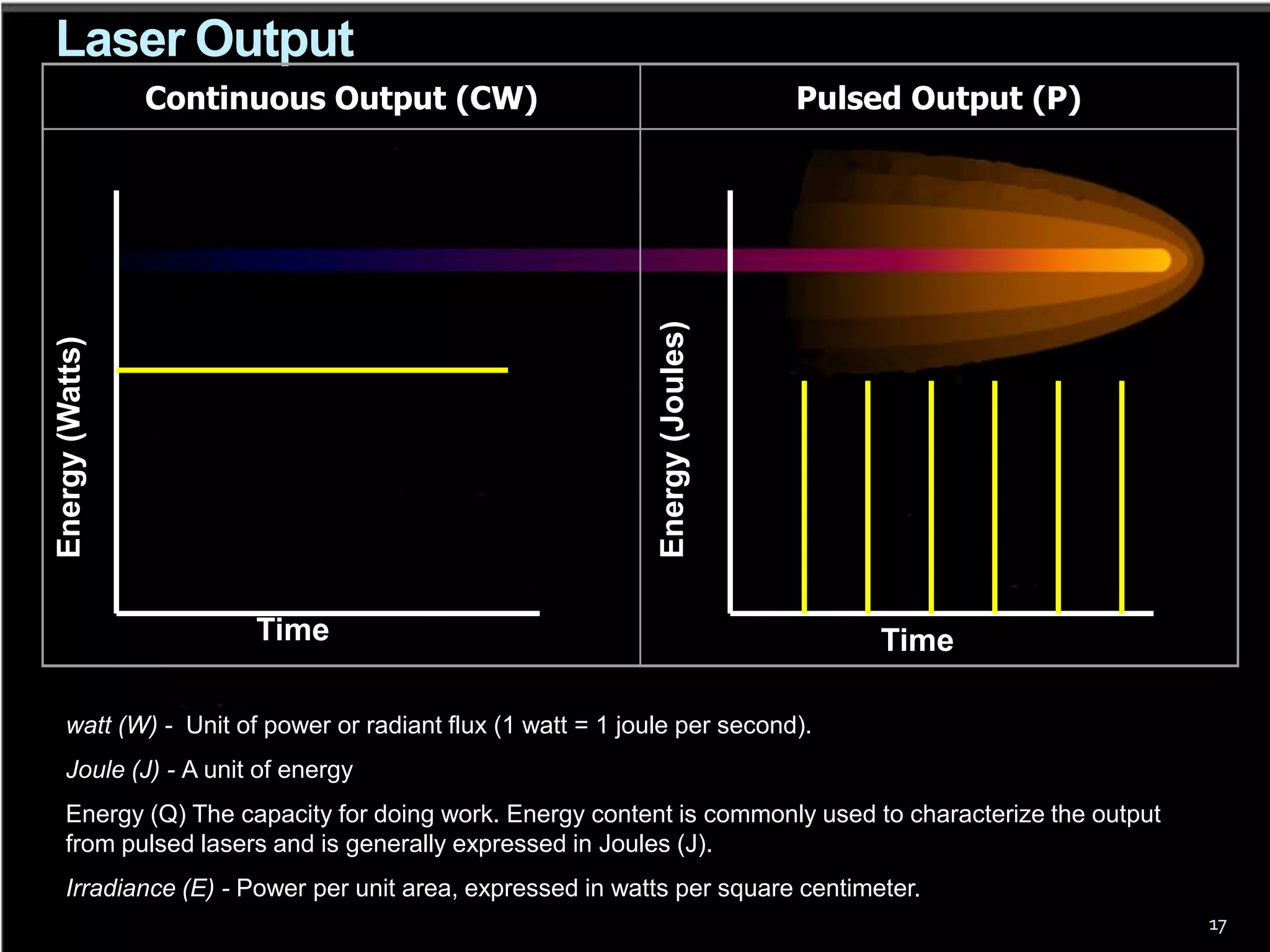
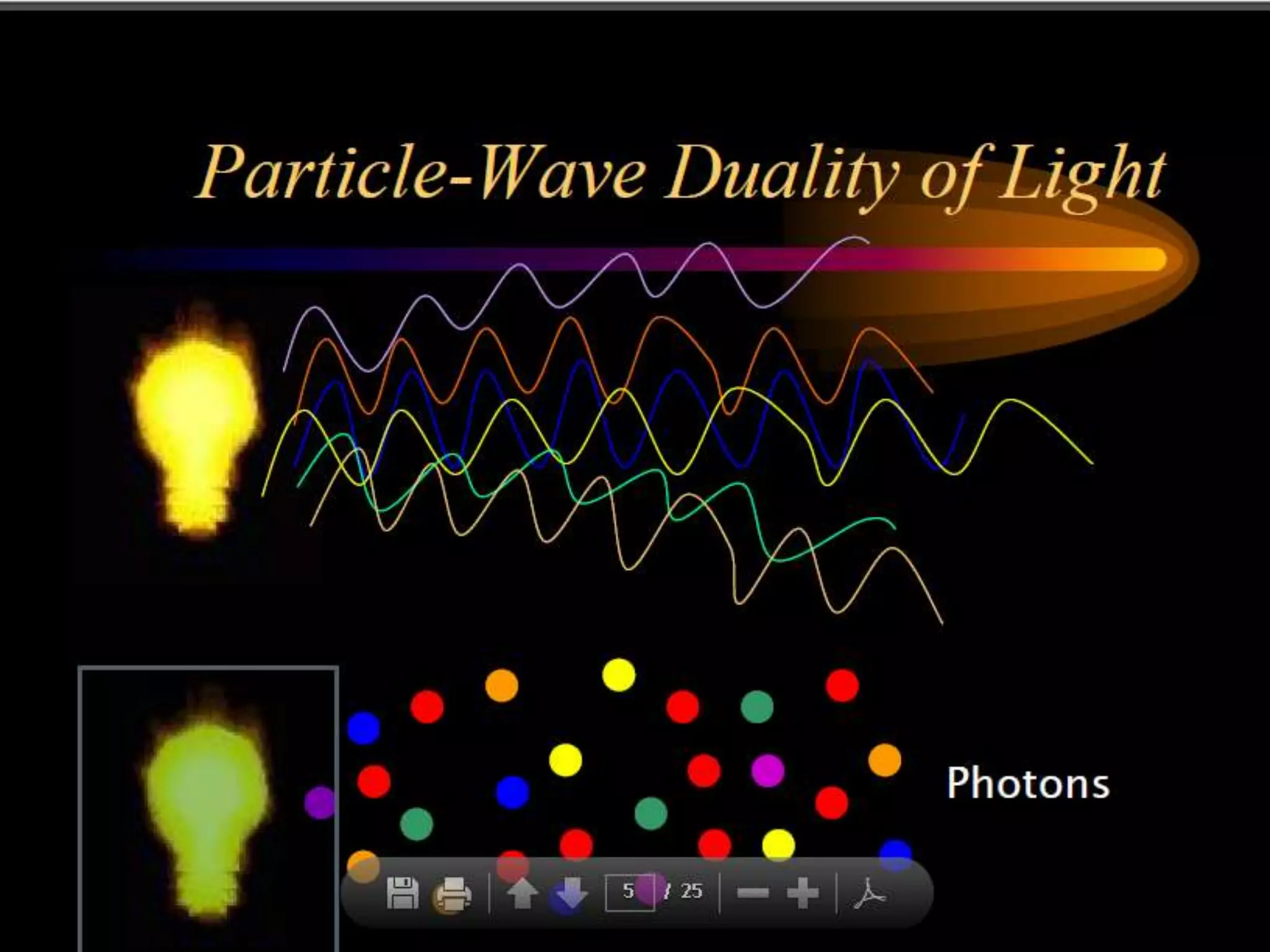
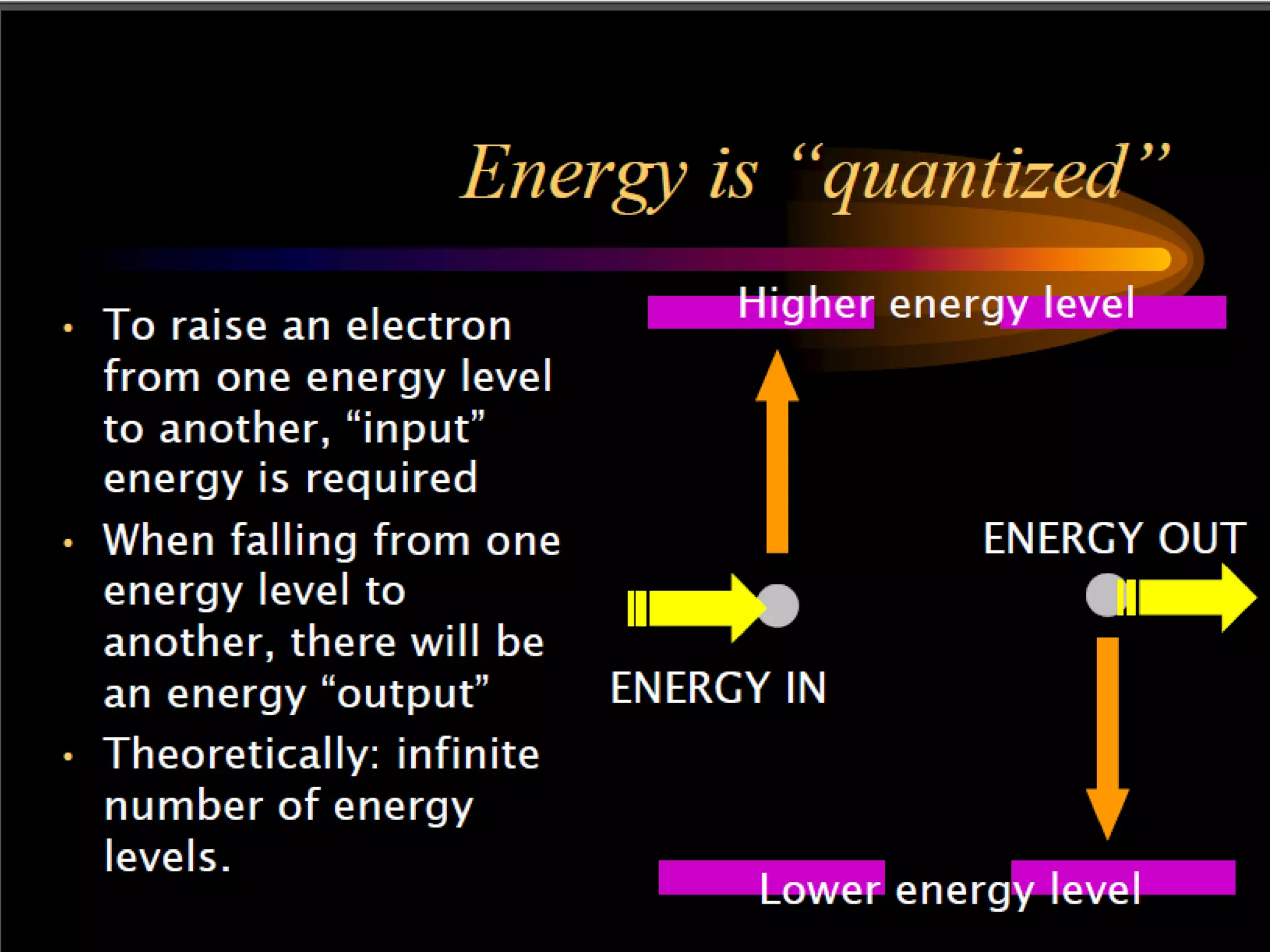
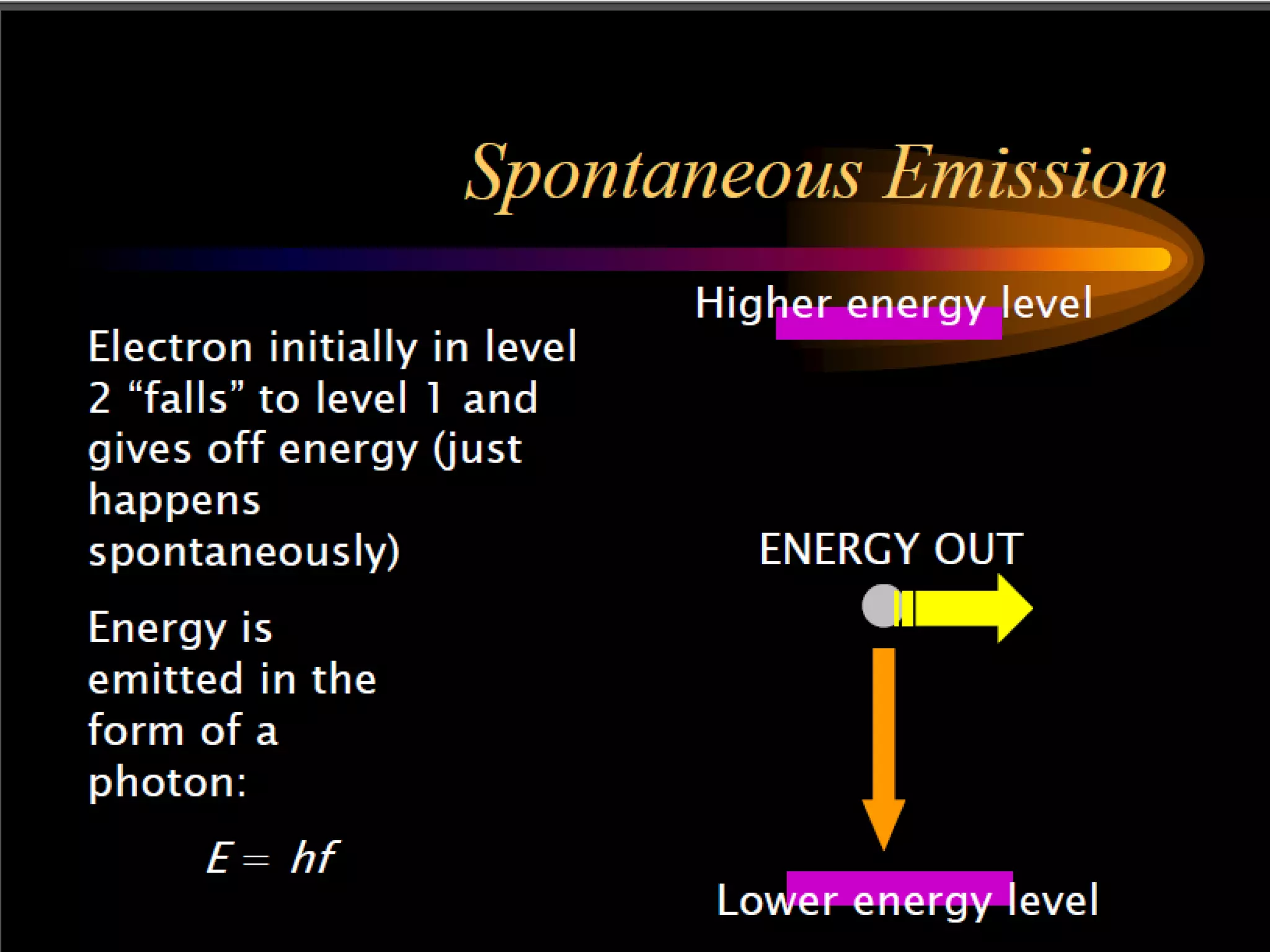
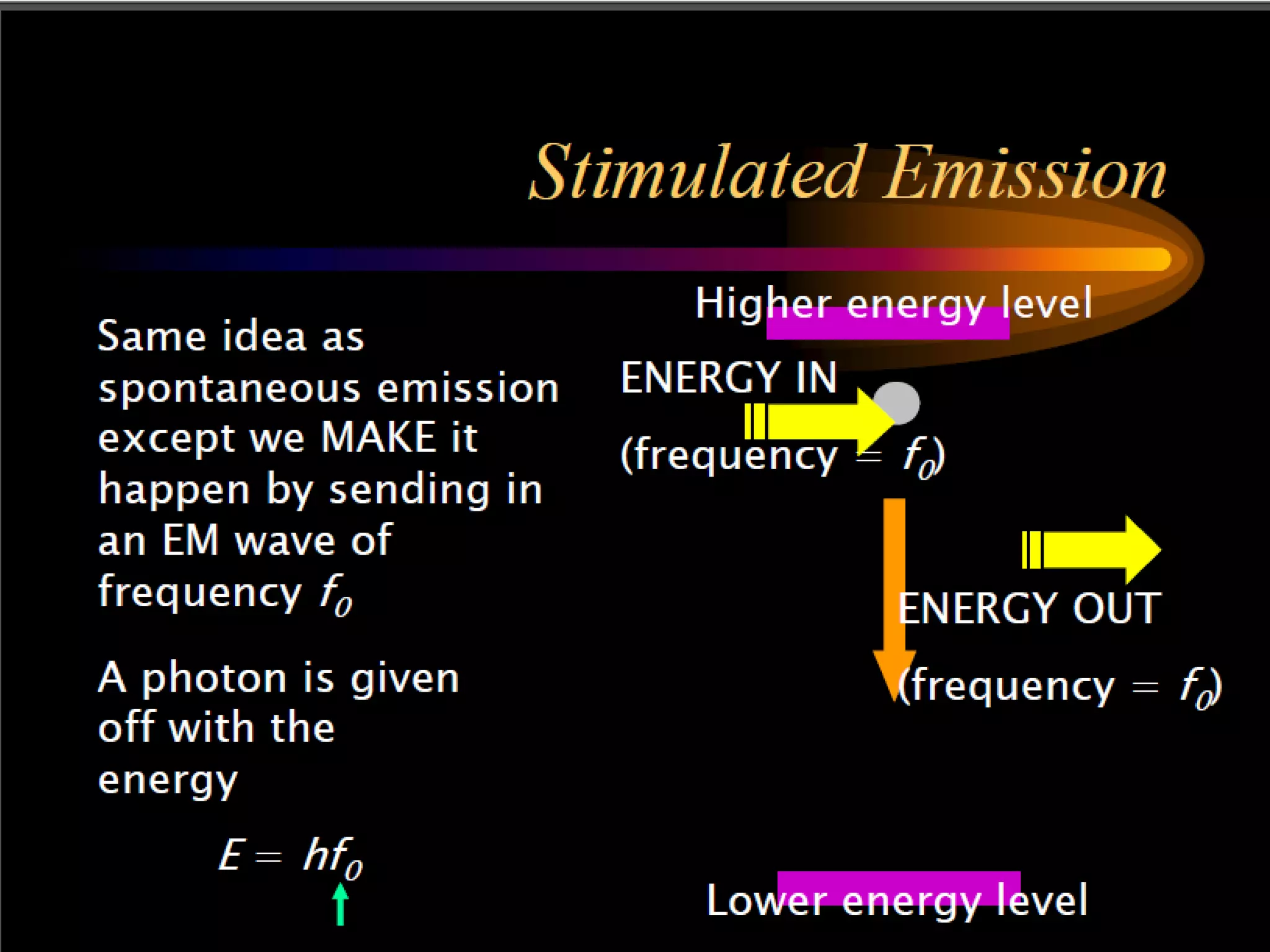
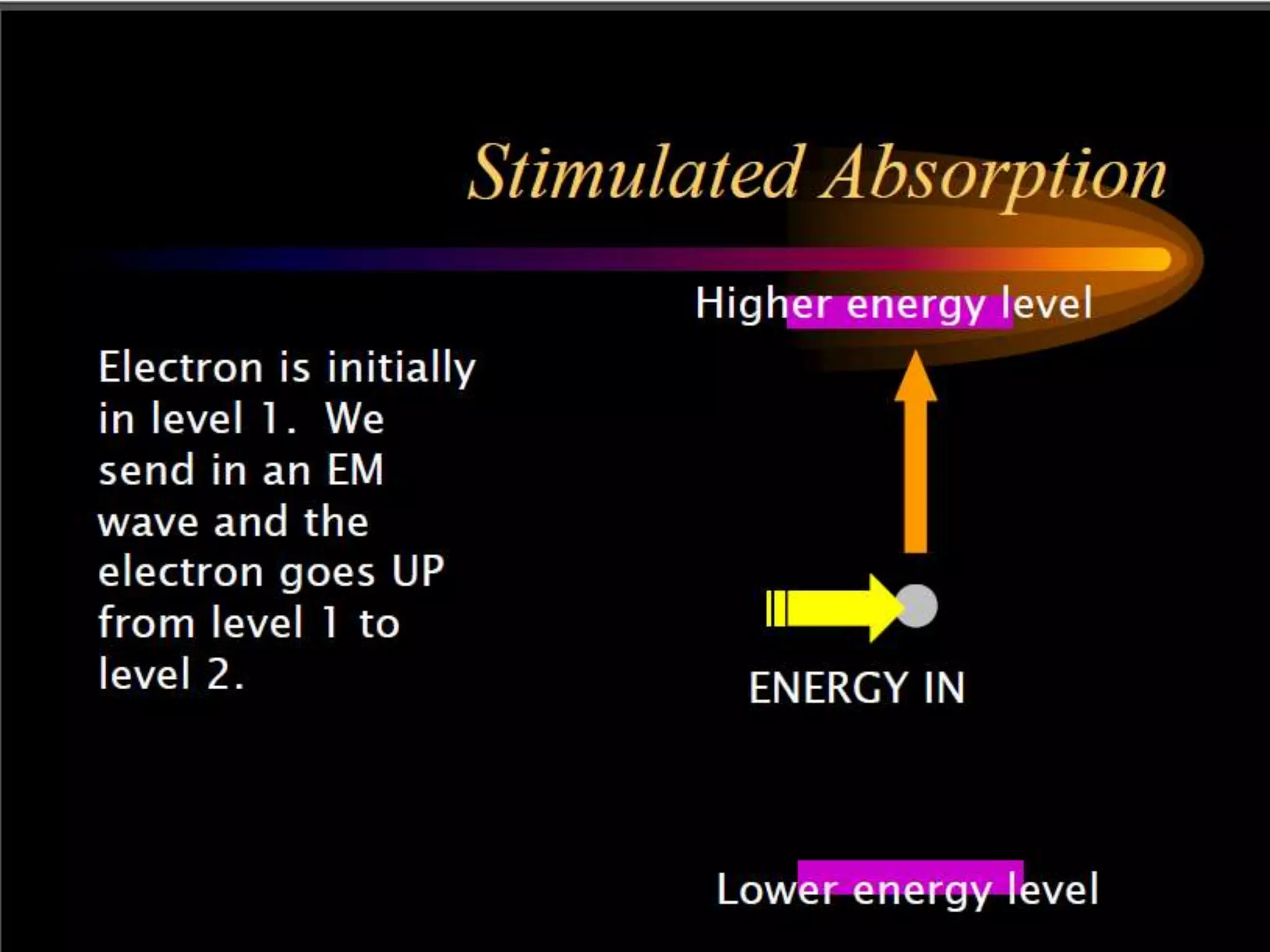
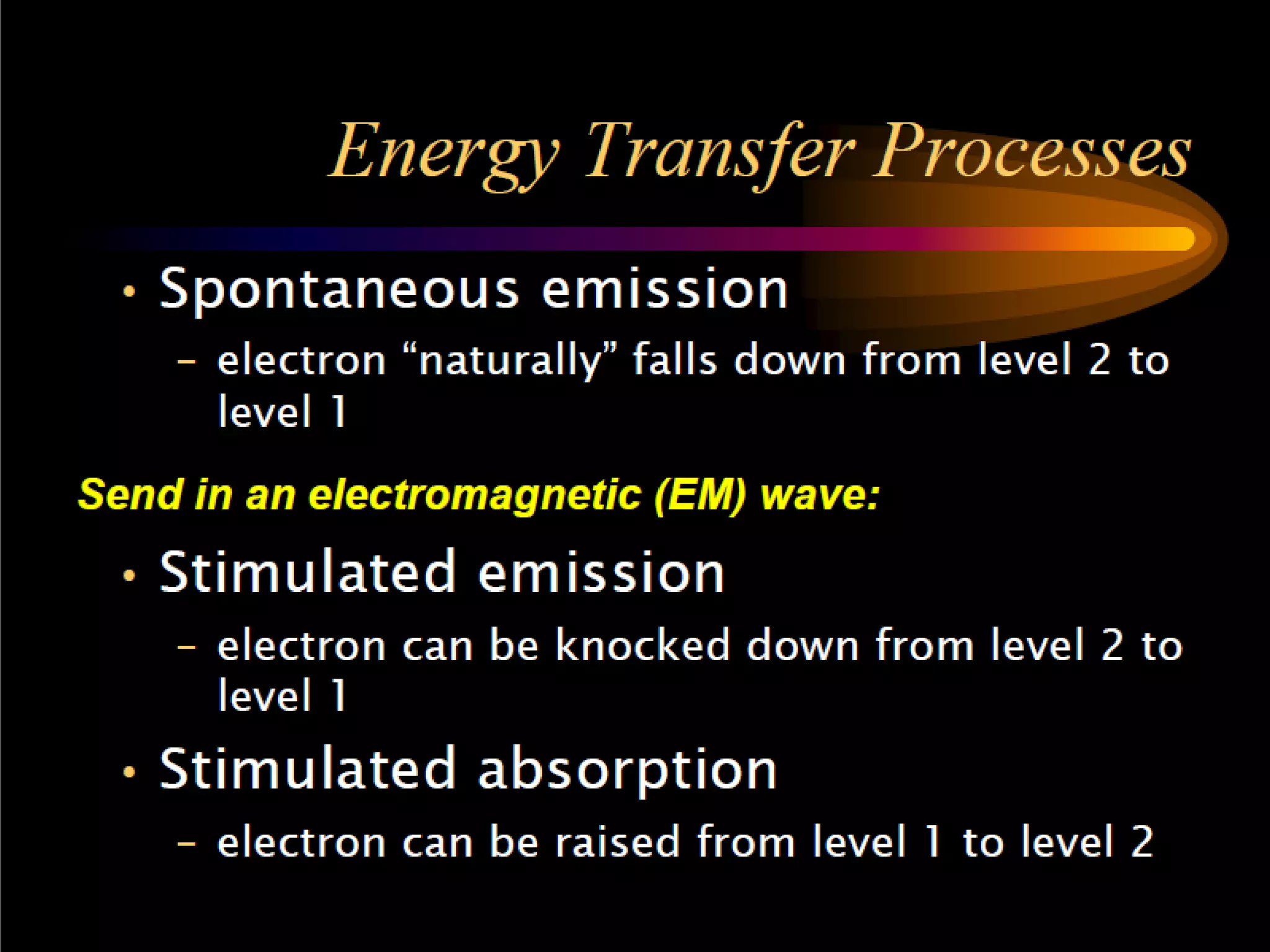
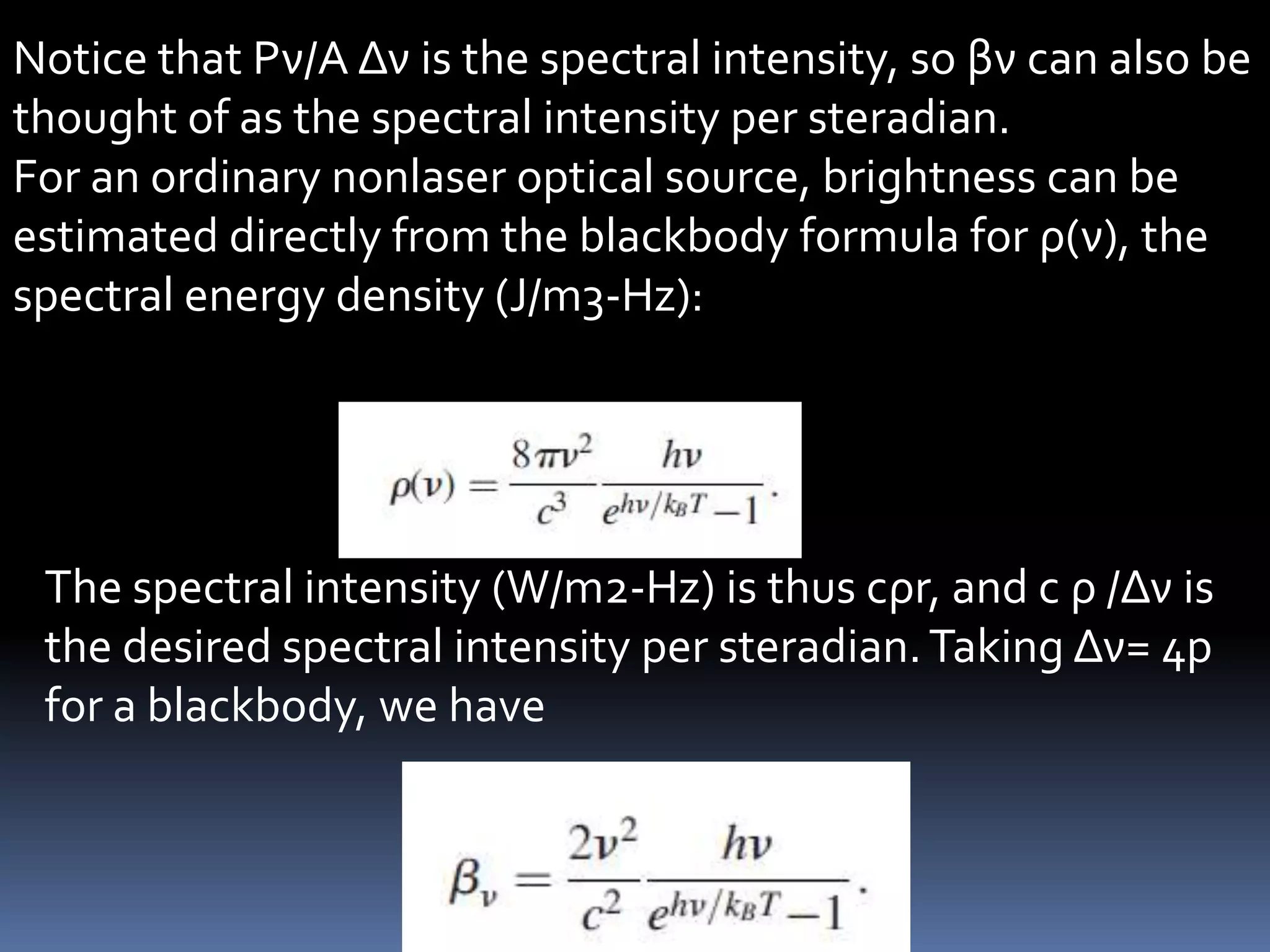
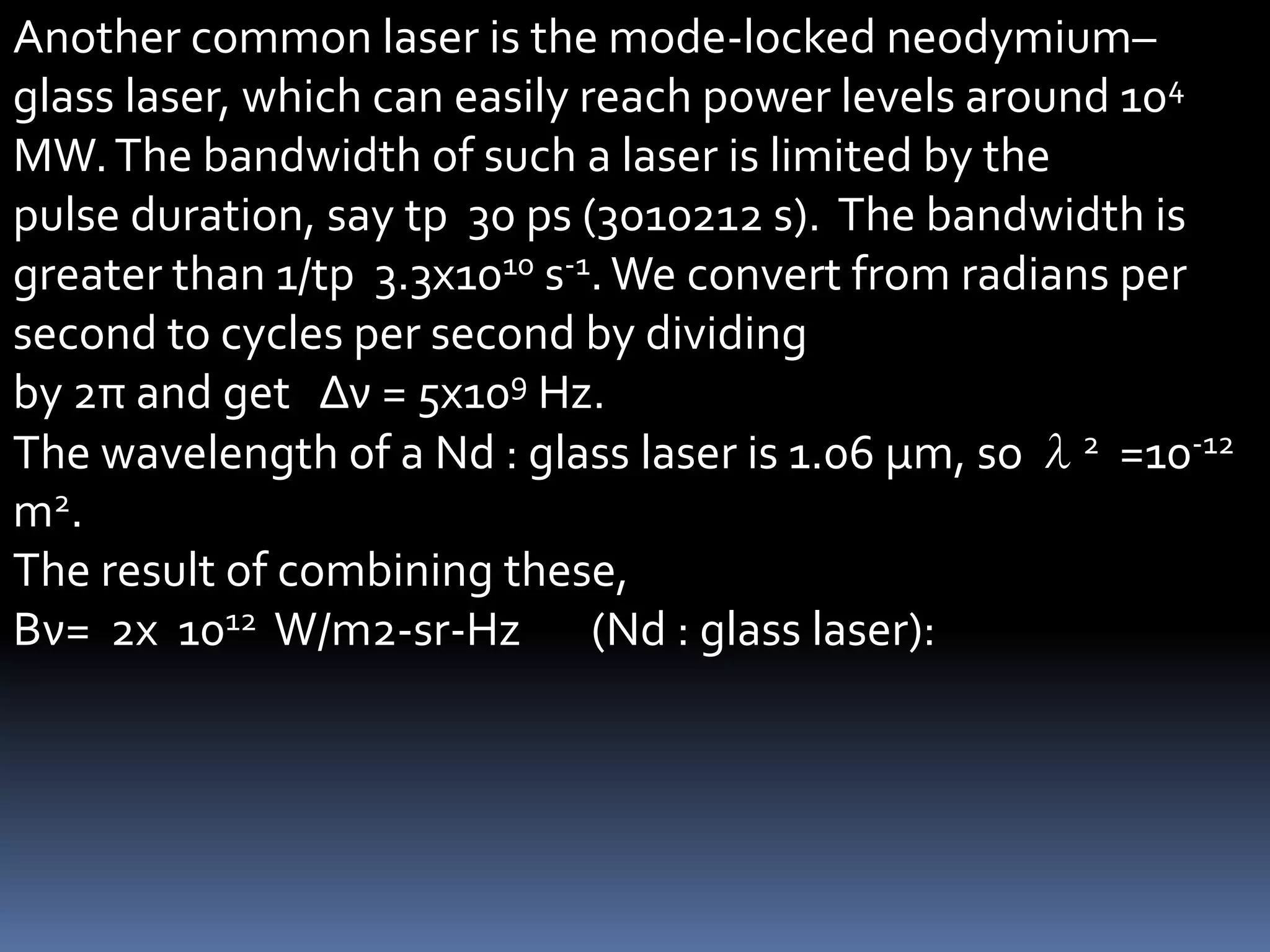
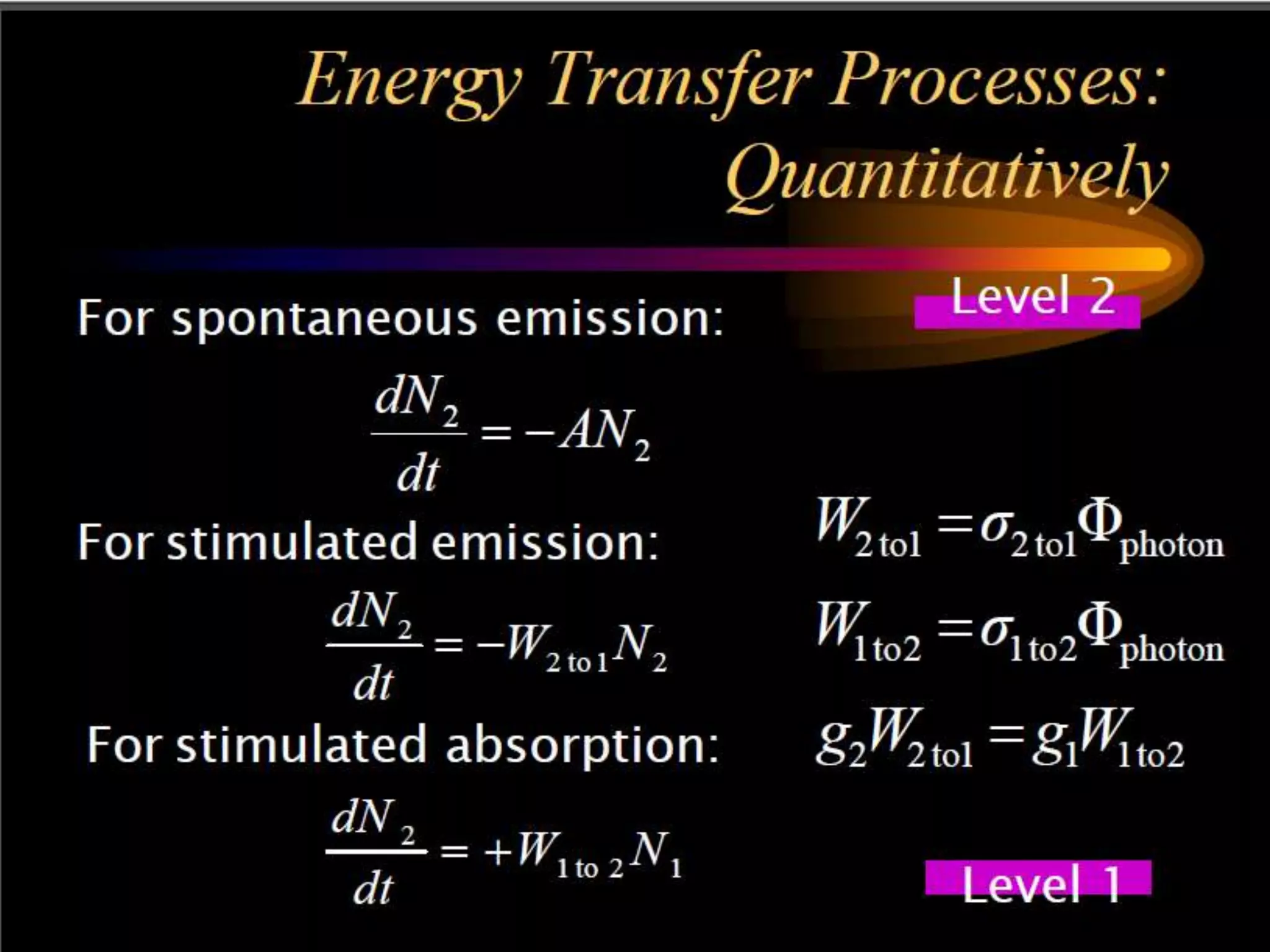
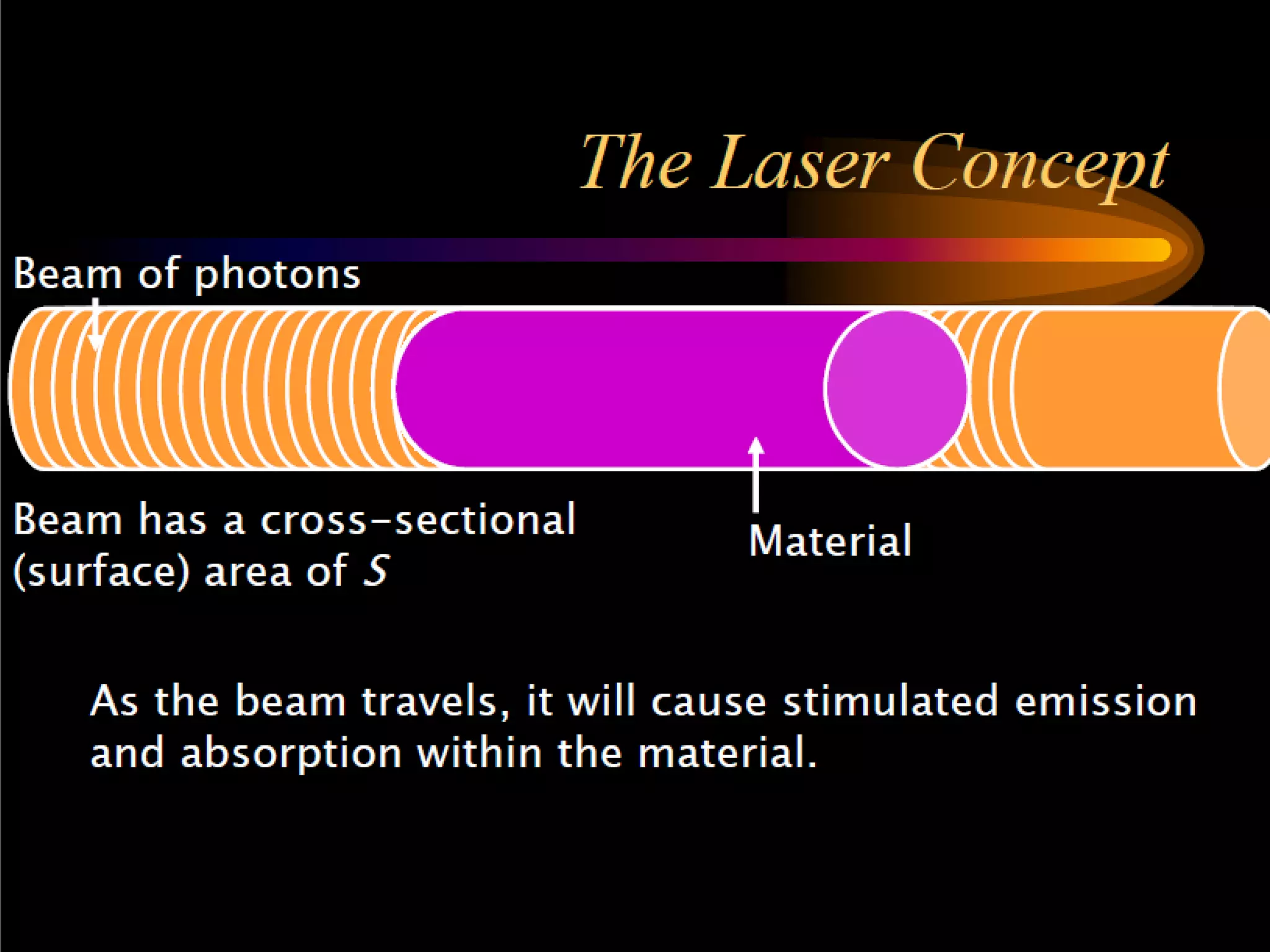
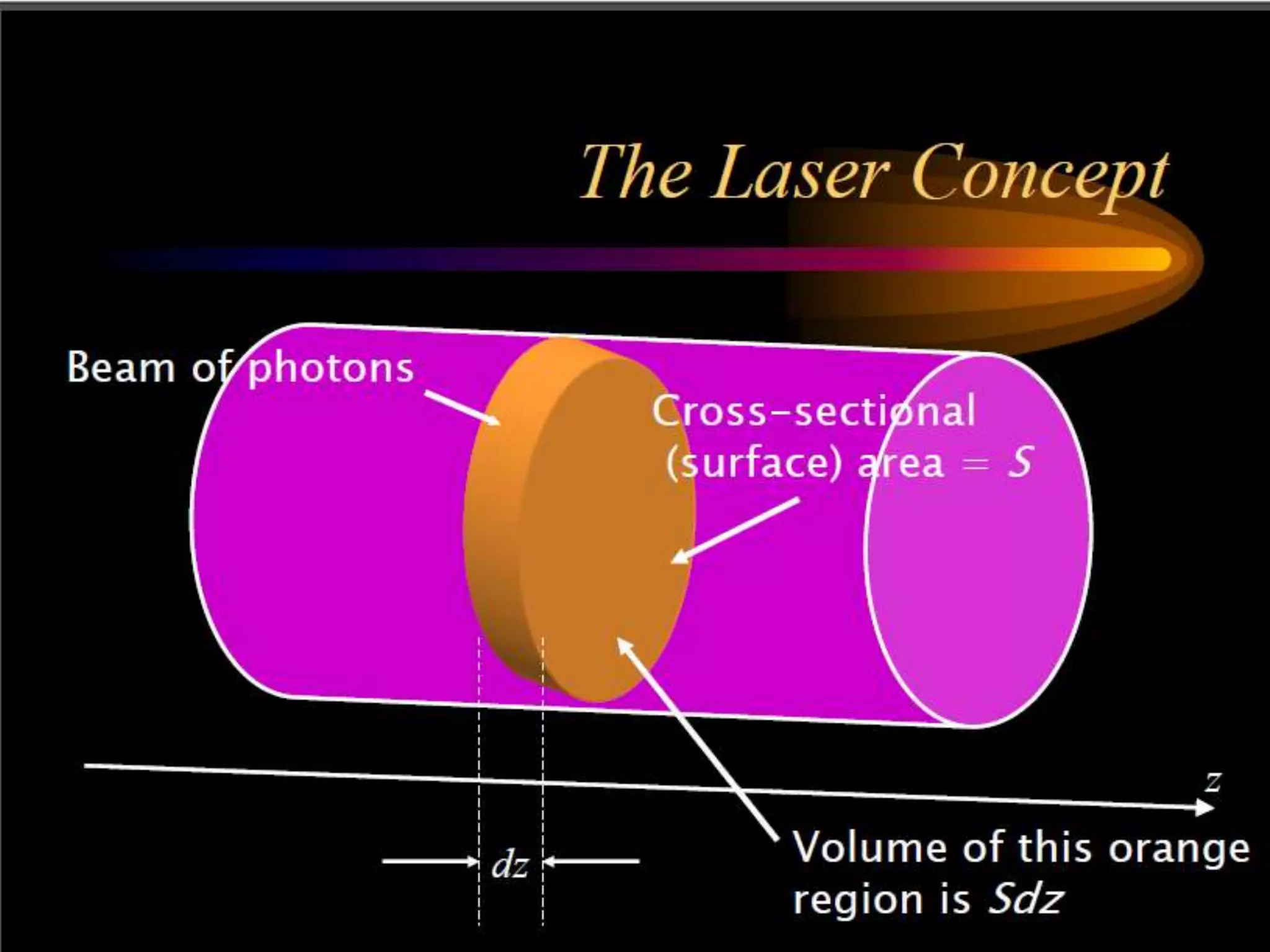
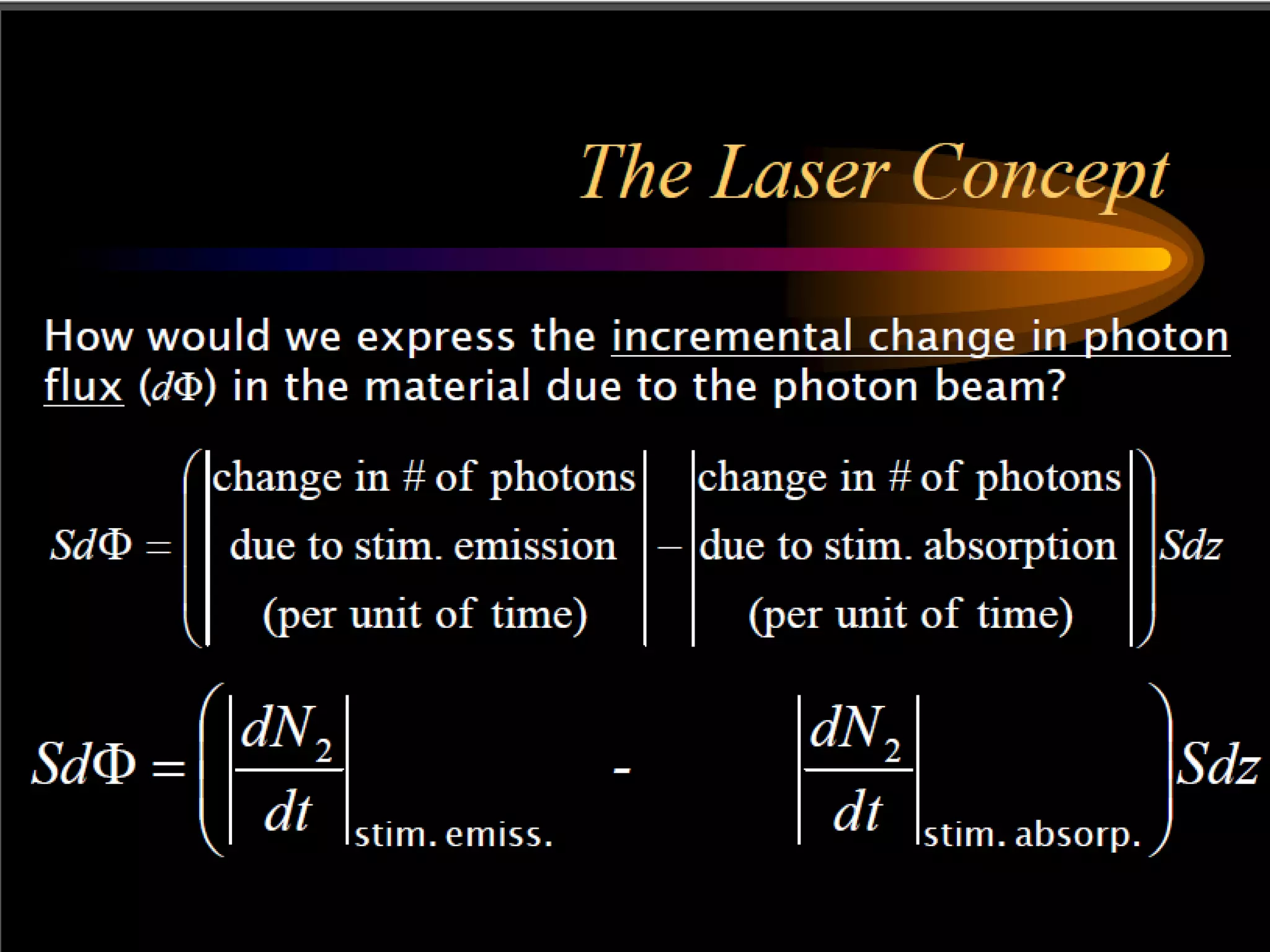
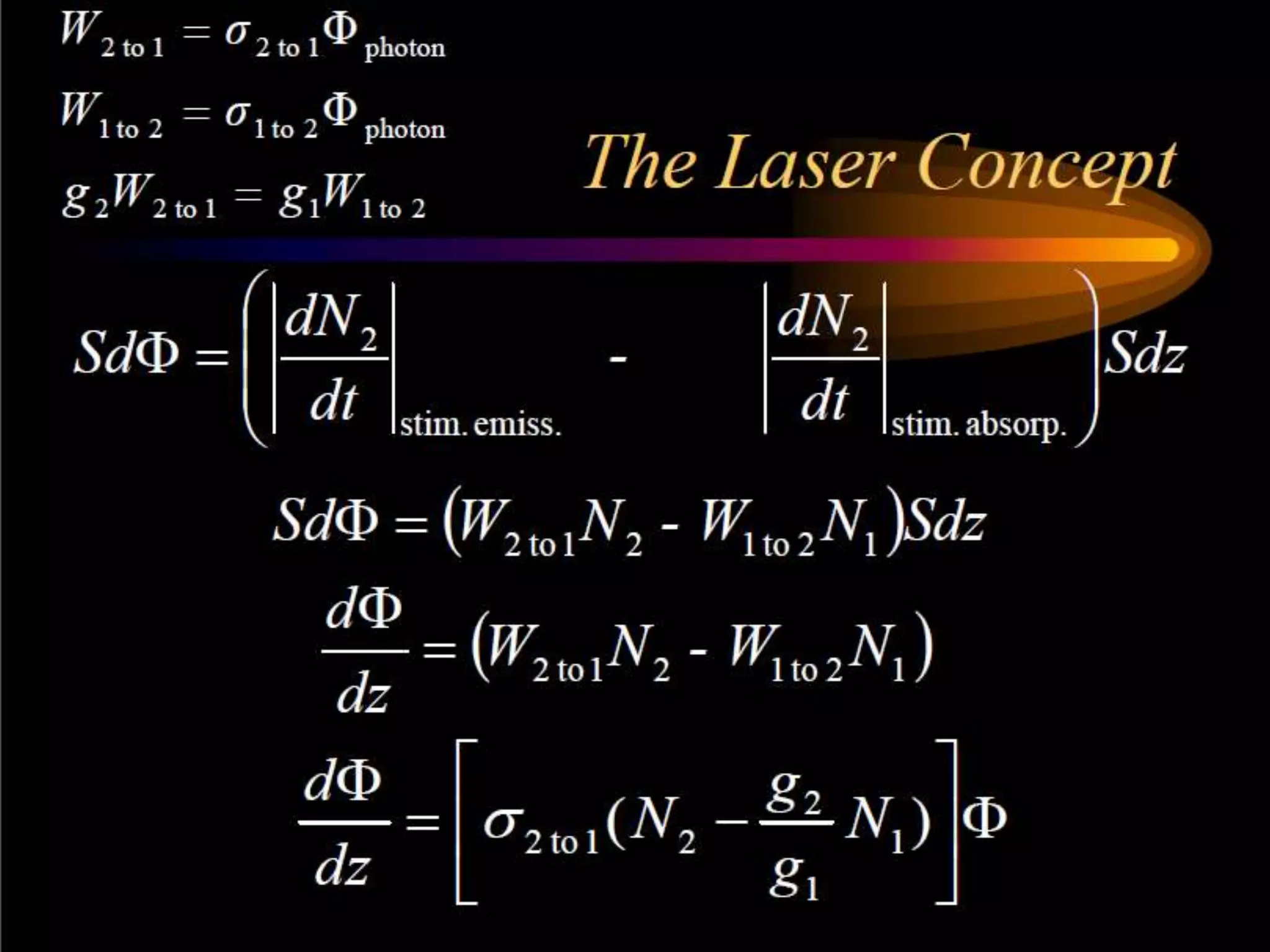
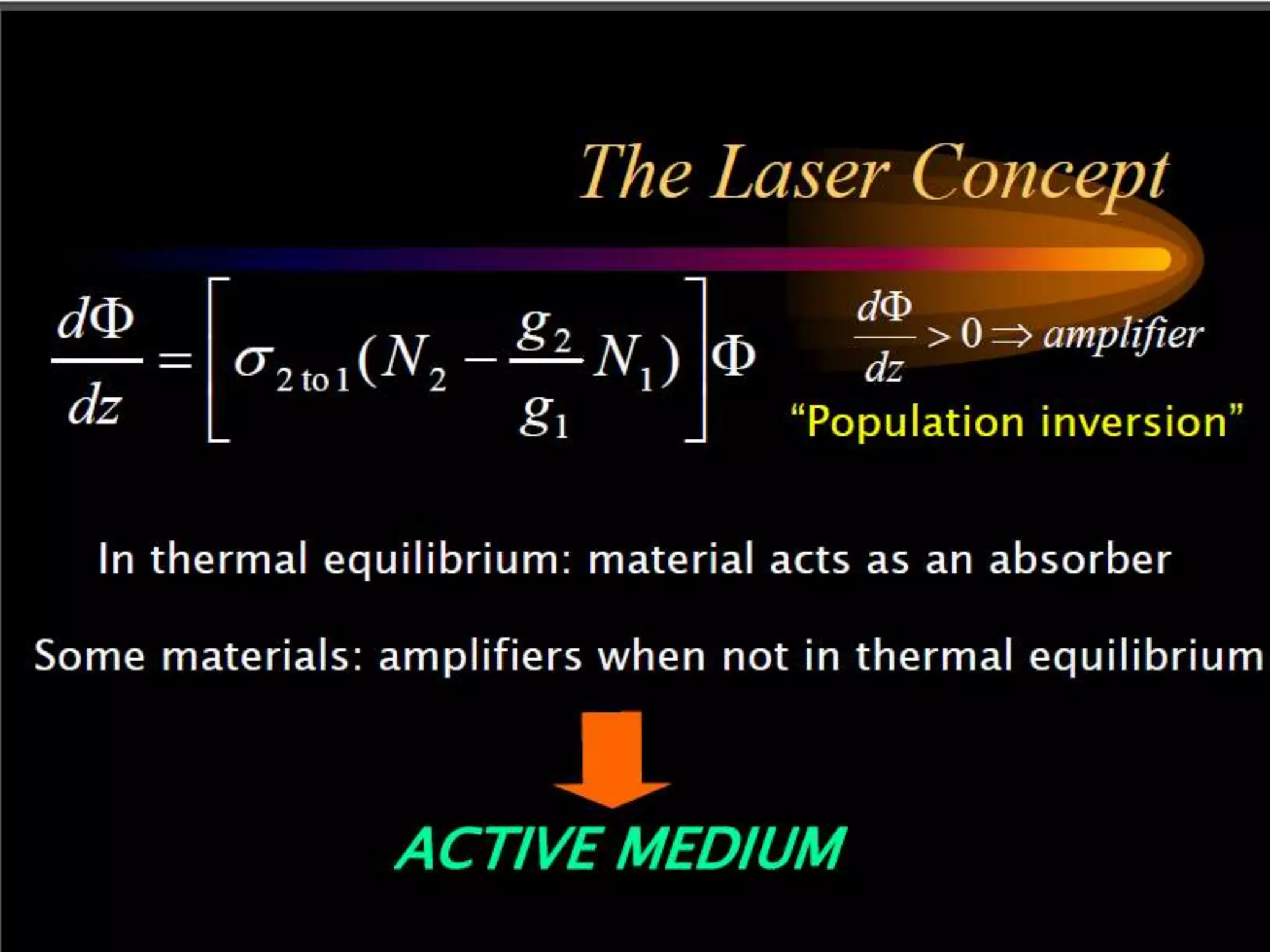
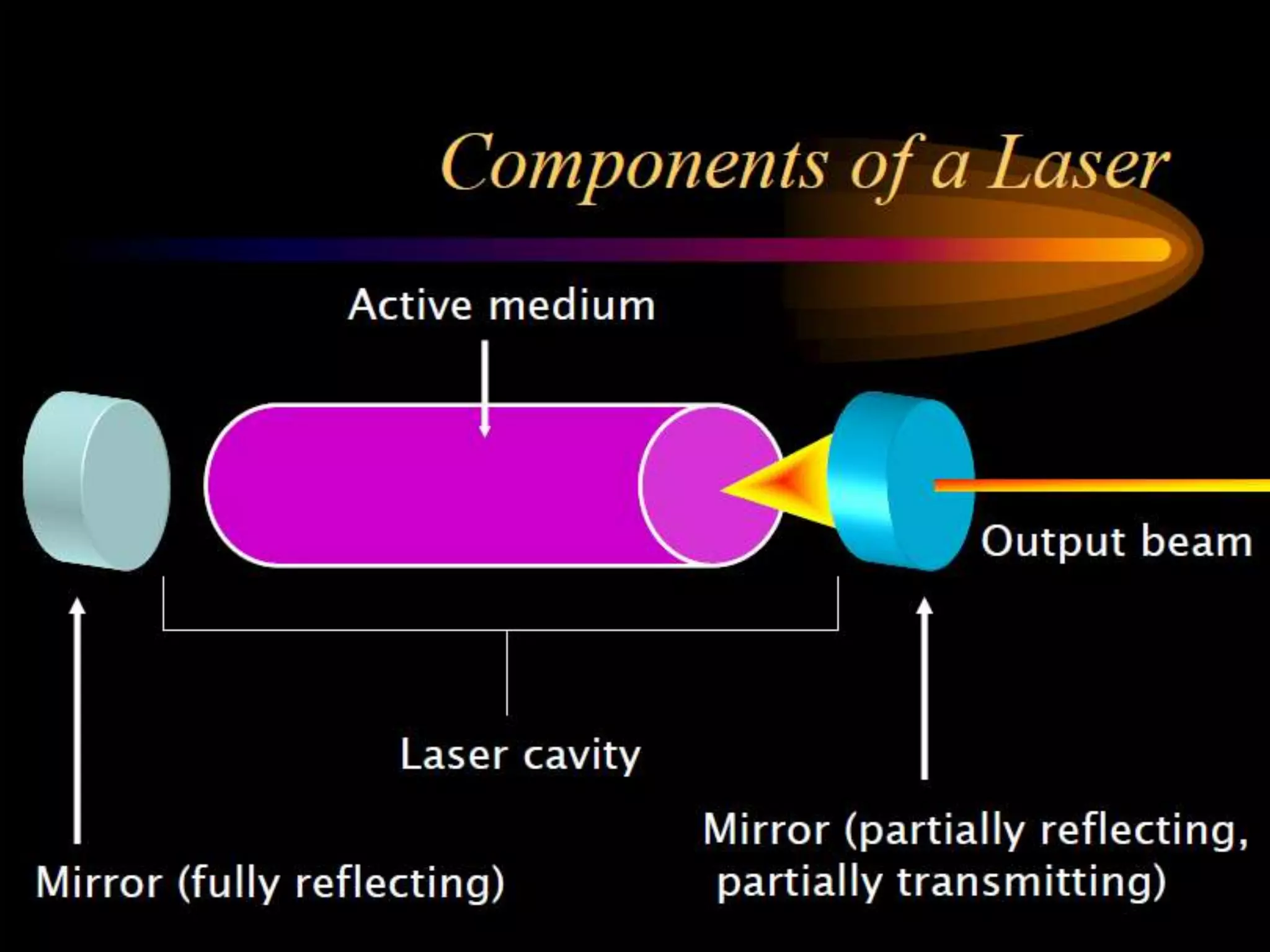
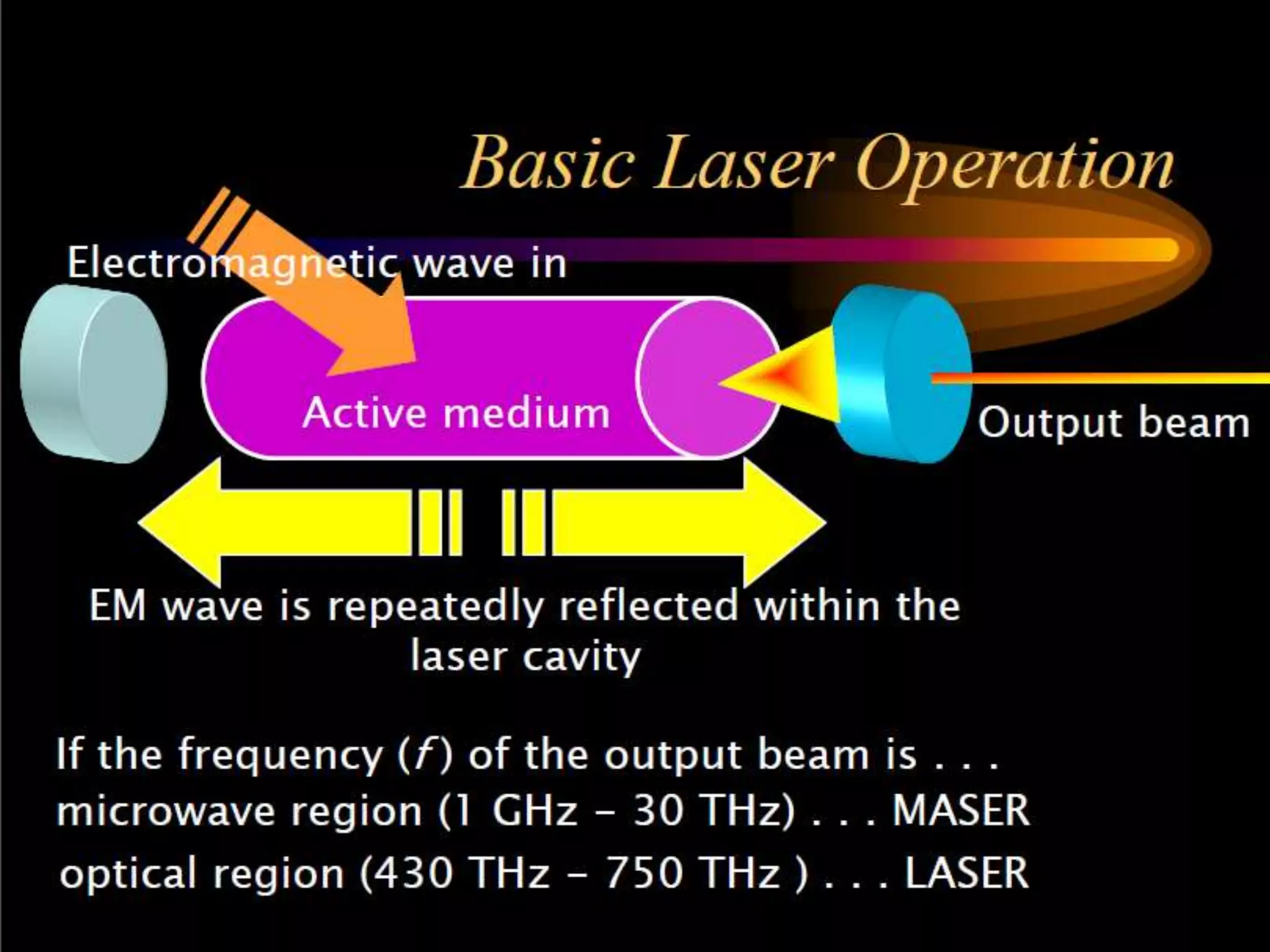
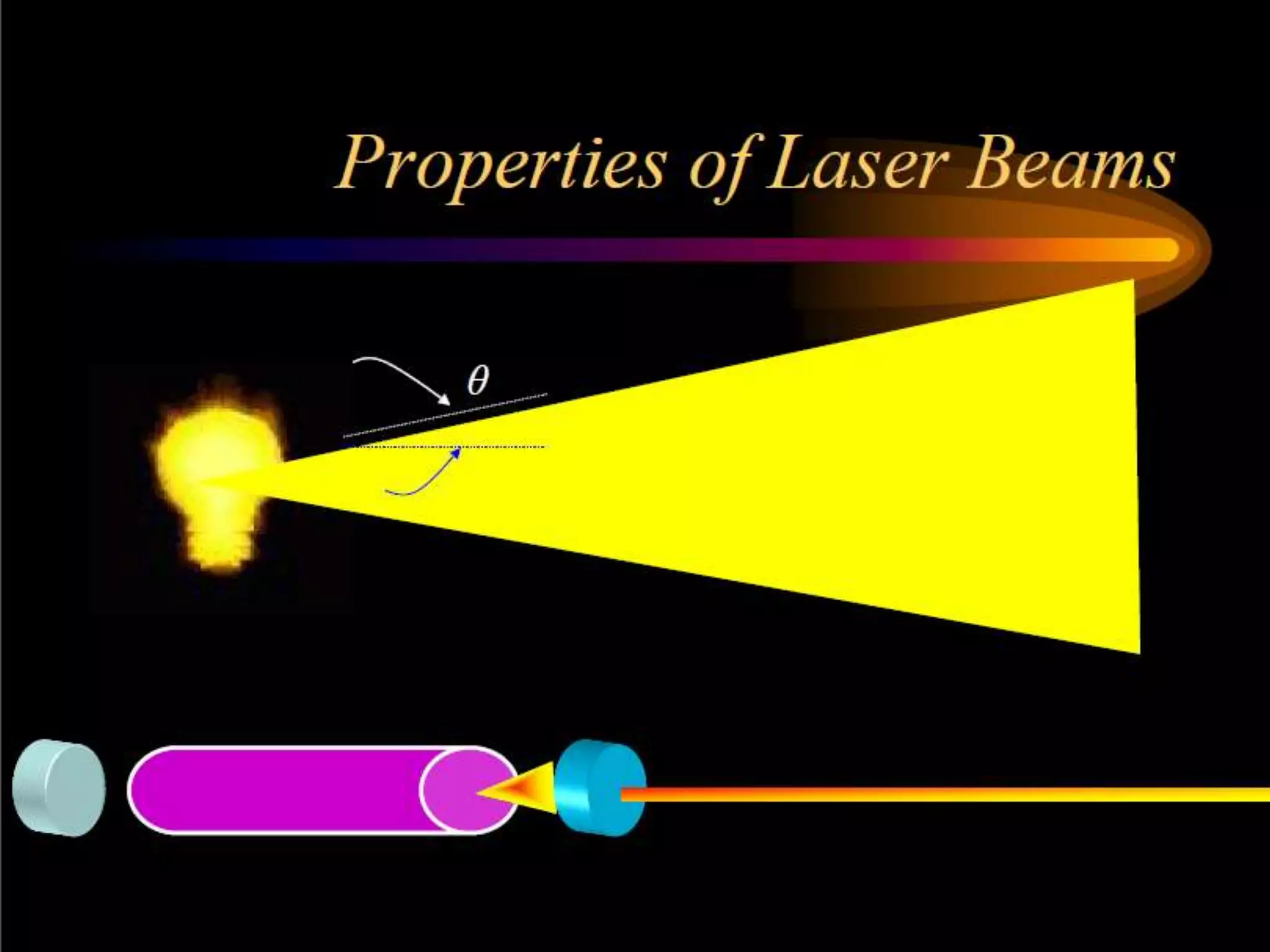
This document outlines the course structure and content for an introduction to laser theory class. The course will include 12 lectures, 4 homework assignments, a midterm exam, final exam, and individual reports. Key topics that will be covered include laser fundamentals, energy levels, rate equations, cavity design, gas lasers, solid state lasers, semiconductor lasers, and other laser types. Lasers can be classified based on their operation mode, population inversion mechanism, or active medium used. The goal is for students to understand the basic scientific principles that enable laser operation.