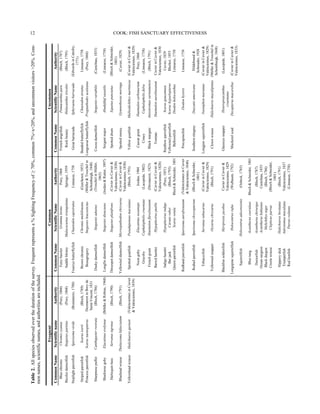
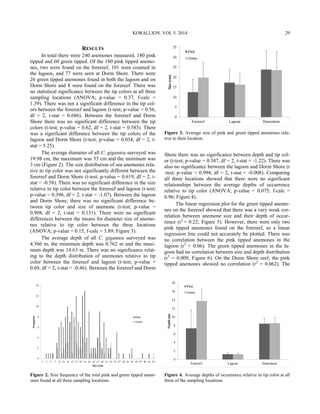
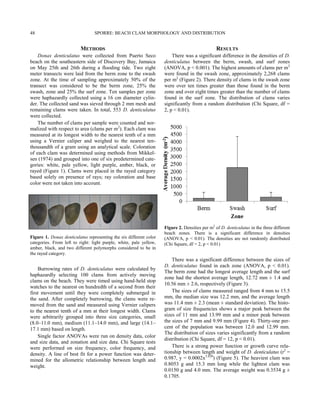
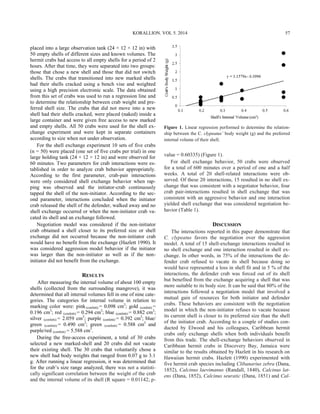
This study examined the population density and depth distribution of the long-spined sea urchin Diadema antillarum in Discovery Bay, Jamaica. D. antillarum is a keystone species that regulates algal growth on coral reefs through grazing. The study found that D. antillarum densities were highest between 5-15 meters deep, defining the "Diadema zone." Compared to 2010, D. antillarum populations have increased in 2014 and the depth range of highest densities has become slightly shallower. The recovery of D. antillarum is important for the health of Caribbean coral reefs by limiting competition between algae and corals for space and light.