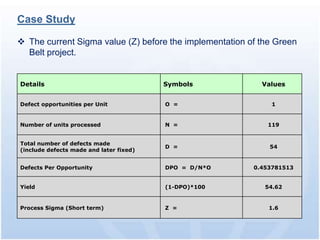
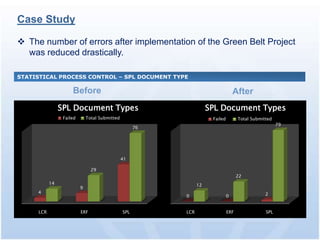
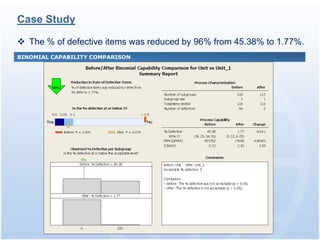
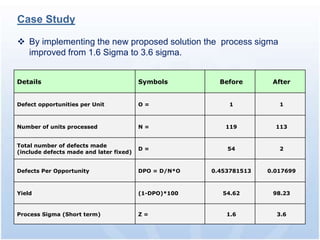
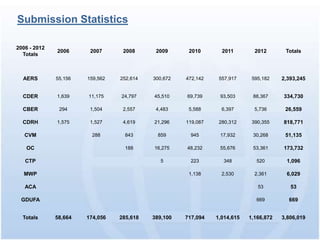
The document discusses common regulatory issues and challenges with electronic submissions to the FDA's Electronic Submissions Gateway (ESG). It notes that while ESG streamlines submissions, users often face validation issues that result in failed submissions and resubmissions. This increases costs and processing time. The document presents a case study that used process improvement methods to increase a vendor's SPL submission success rate from 54% to over 98%. It concludes that analyzing submission failures across all FDA centers could further improve the electronic submission process.