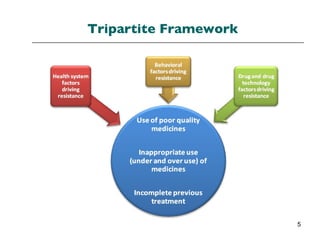
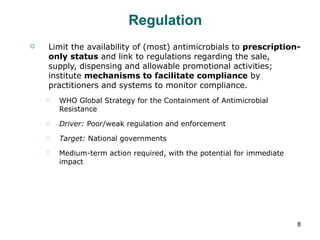
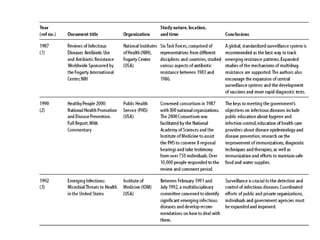
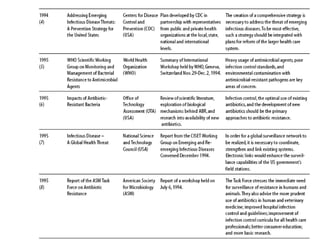
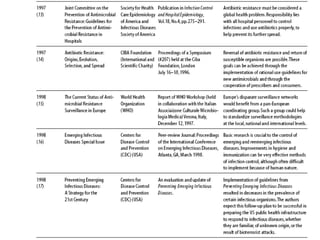
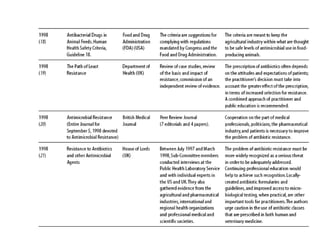
The document summarizes recommendations from various sources to address the problem of antimicrobial resistance. It outlines recommendations in three areas: health systems, behavior changes, and technology developments. For each area, it lists specific recommendations, the organizations that proposed them, and considerations around implementation such as targeted stakeholders and timeframes. Key recommendations include improving regulation and surveillance of antimicrobial use, optimizing treatment guidelines, educating providers and patients, developing new diagnostics and drugs, and providing incentives for research and development.