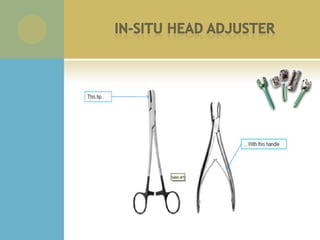
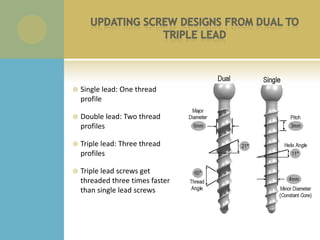
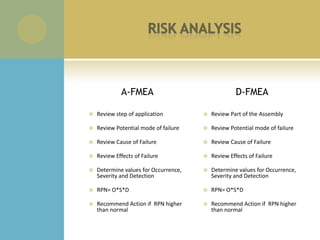
This document summarizes the activities of an orthopedic product company with three divisions: orthopedic, spine, and sports medicine. It describes new product development phases from initial concept to user preference evaluation. It also outlines engineering projects including custom instrumentation, screw design changes, and pull out strength testing. Regulatory documents and quality systems like risk analysis and FMEAs are referenced. Recommended skills for medical device engineering interns are listed.