- The document is an investor presentation for IntelGenx Corp from April 2018.
- It provides an overview of IntelGenx, including its business strategies, product pipeline, clinical trial results, manufacturing facilities, and leadership team.
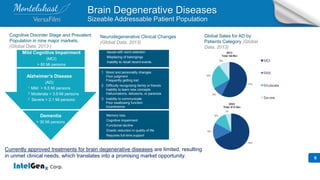
- The presentation highlights IntelGenx's drug delivery technology platforms, focus on developing generic and repurposed products, and partnerships with pharmaceutical companies.