Embed presentation
Download to read offline



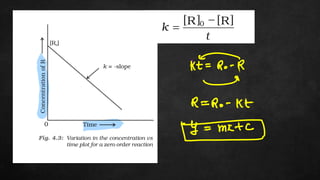



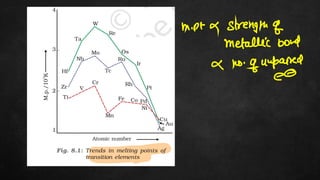




The document discusses several chemistry concepts including: 1) Adsorption isotherms and how they relate absorption to concentration. 2) Enthalpy changes associated with various chemical processes like atomization, nuclear charge effects, and ionization. 3) Trends in various enthalpy values like sublimation, hydration, and ionization across periods in the periodic table.











