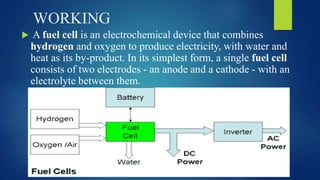
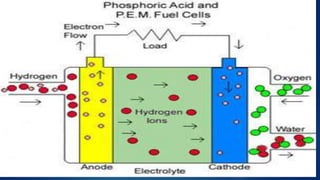
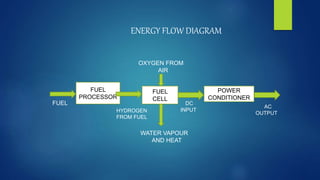
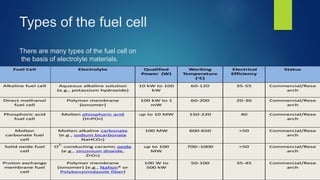
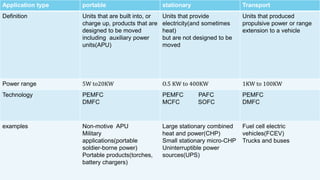
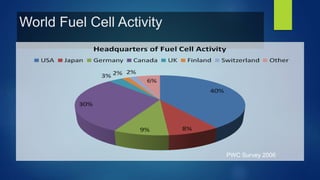
The document provides an overview of hydrogen-based fuel cell technology. It discusses the history of fuel cells from their invention in 1839 to current applications in vehicles. The document outlines the working of a fuel cell and explains why fuel cell technology is needed to reduce air pollution and dependence on fossil fuels. It also discusses challenges like high costs, lack of infrastructure and durability issues that need to be addressed for widespread adoption of fuel cells.