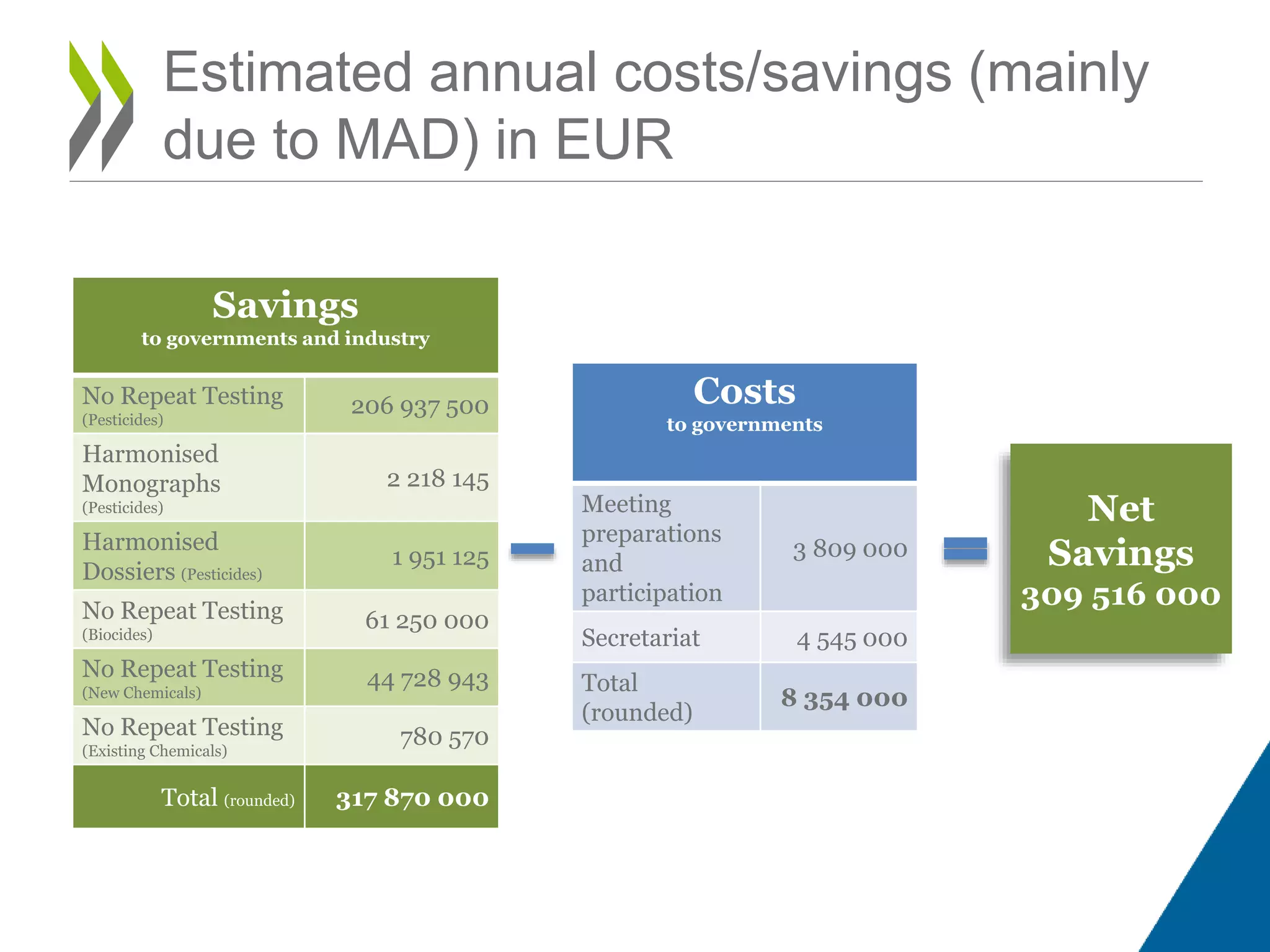
The document discusses OECD's Mutual Acceptance of Data (MAD) system, which aims to avoid duplicative testing of chemicals by industry and reduce non-tariff trade barriers. Under MAD, toxicological and ecotoxicological data generated in OECD countries in accordance with OECD Test Guidelines and Good Laboratory Practice principles must be accepted by other OECD members for regulatory purposes. Approximately 160 Test Guidelines cover various endpoints. Guidelines are regularly updated to meet regulatory needs. MAD is estimated to result in over 300 million euros in annual net savings through eliminating redundant testing.