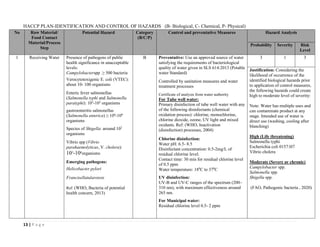
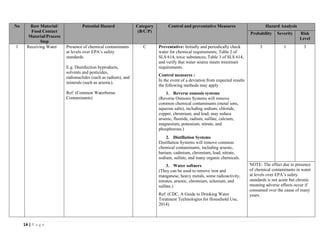
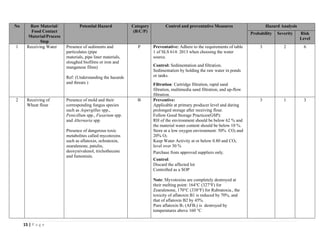
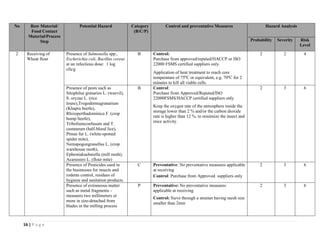
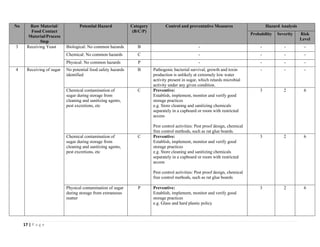
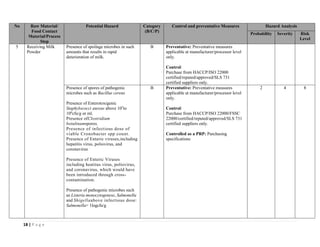
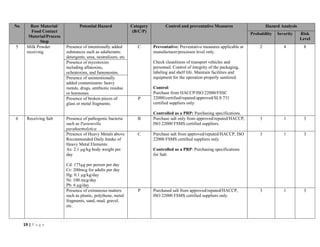
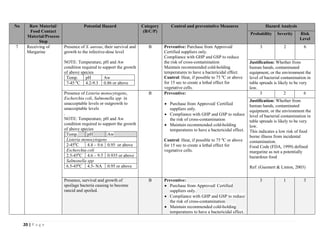
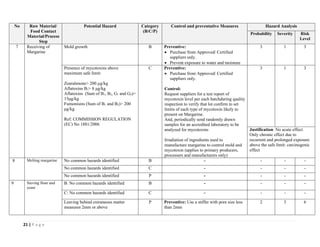
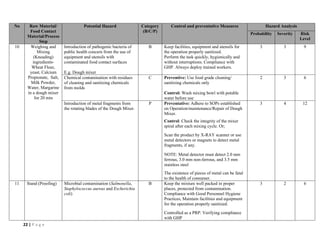
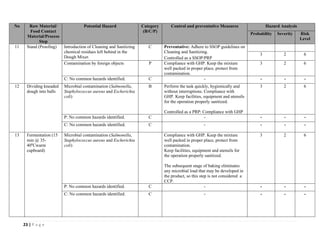
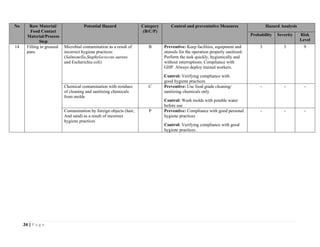
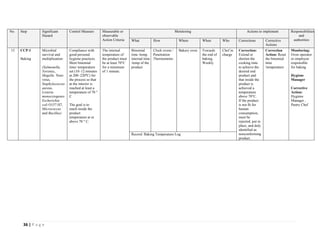
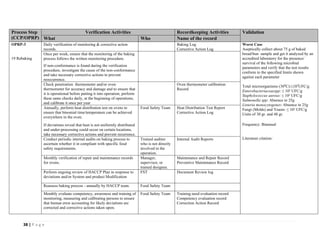
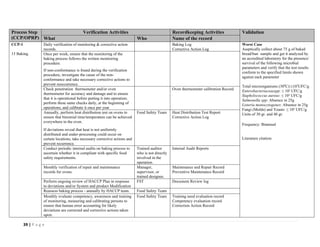
The document outlines a HACCP plan for bread production, detailing the ingredients, production methods, and safety measures to ensure food quality and safety. It includes specifications for raw materials, processing steps, packaging, storage conditions, and microbiological standards, along with risk assessments and contamination control measures. Compliance with relevant regulations and guidelines for food safety is also emphasized throughout the plan.