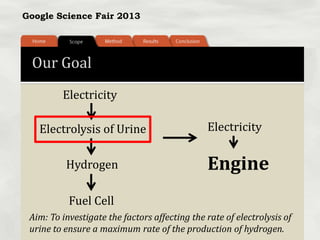
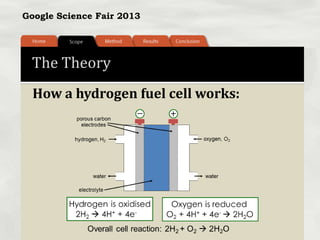
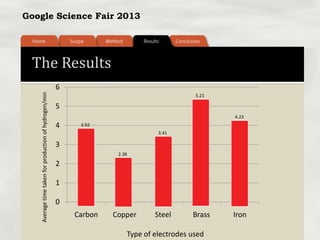
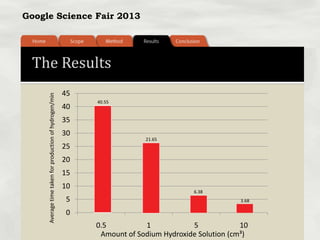
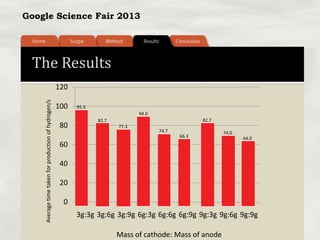
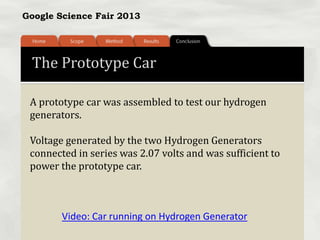
This document summarizes a science fair project that investigated producing hydrogen fuel from urine through electrolysis. The project tested factors that affected the electrolysis rate, including urea concentration, electrode material, alkalinity, and surface area. Optimal conditions were used to construct a hydrogen generator that powered a prototype car, demonstrating the viability of urine as a renewable fuel source.
![Google Science Fair 2013
Chung Ray Ern
Ng Yu Song
Hari Kope
[Teacher Mentor] Mrs Yau Pooi Har
Urine to Fuel
Introductory Video](https://image.slidesharecdn.com/gsffinal3-130429035157-phpapp02/85/Gsf-final-3-1-320.jpg)