Embed presentation
Download to read offline

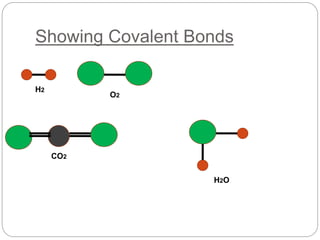

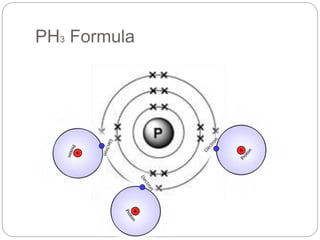

This document discusses writing chemical formulas for covalent substances like H2O, O2, H2, and CO2. It explains that formulas are written by counting the number of atoms of each element, and uses the example of PH3 to show how phosphorus bonds with 3 hydrogen atoms due to its 3 unpaired electrons.





