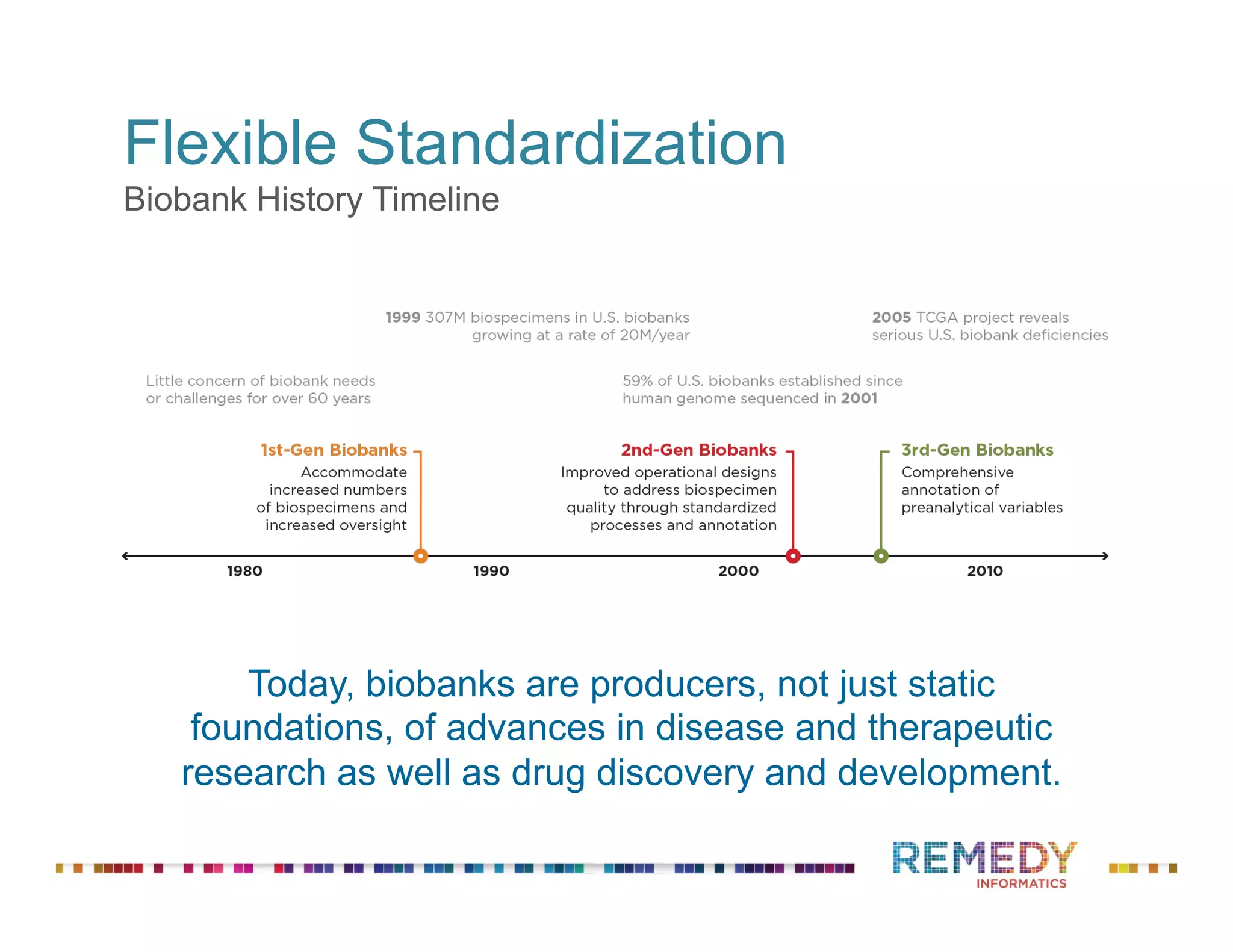
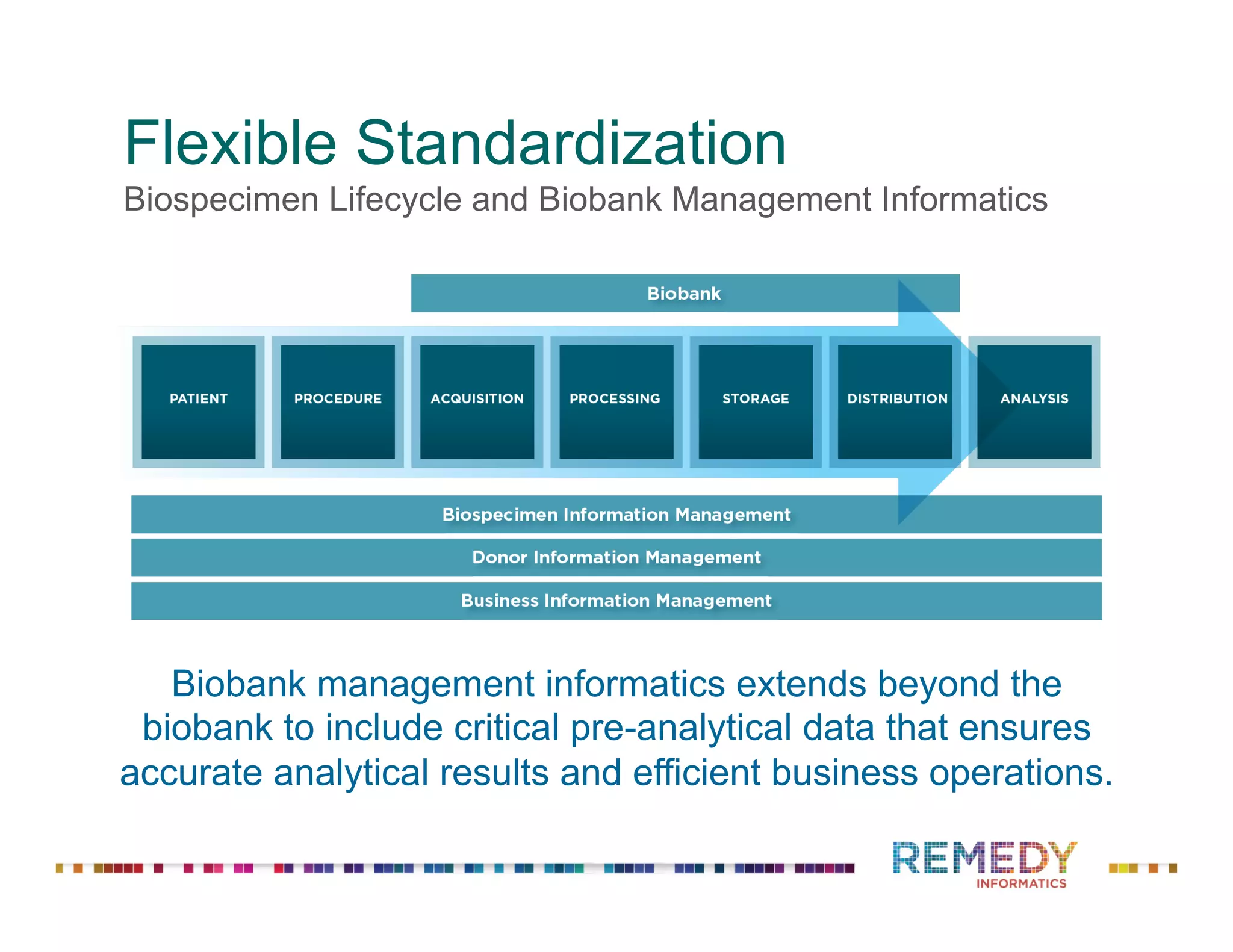
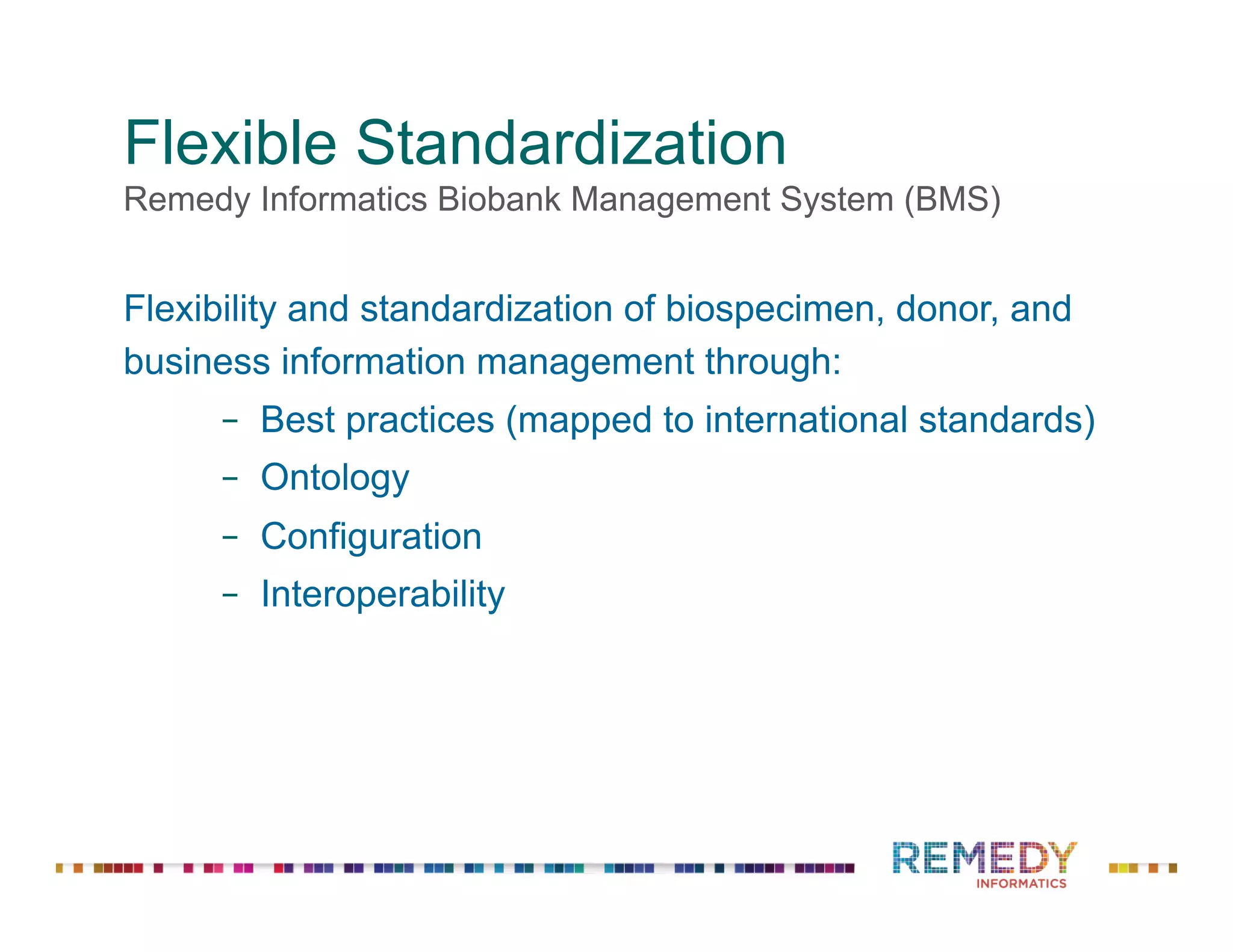
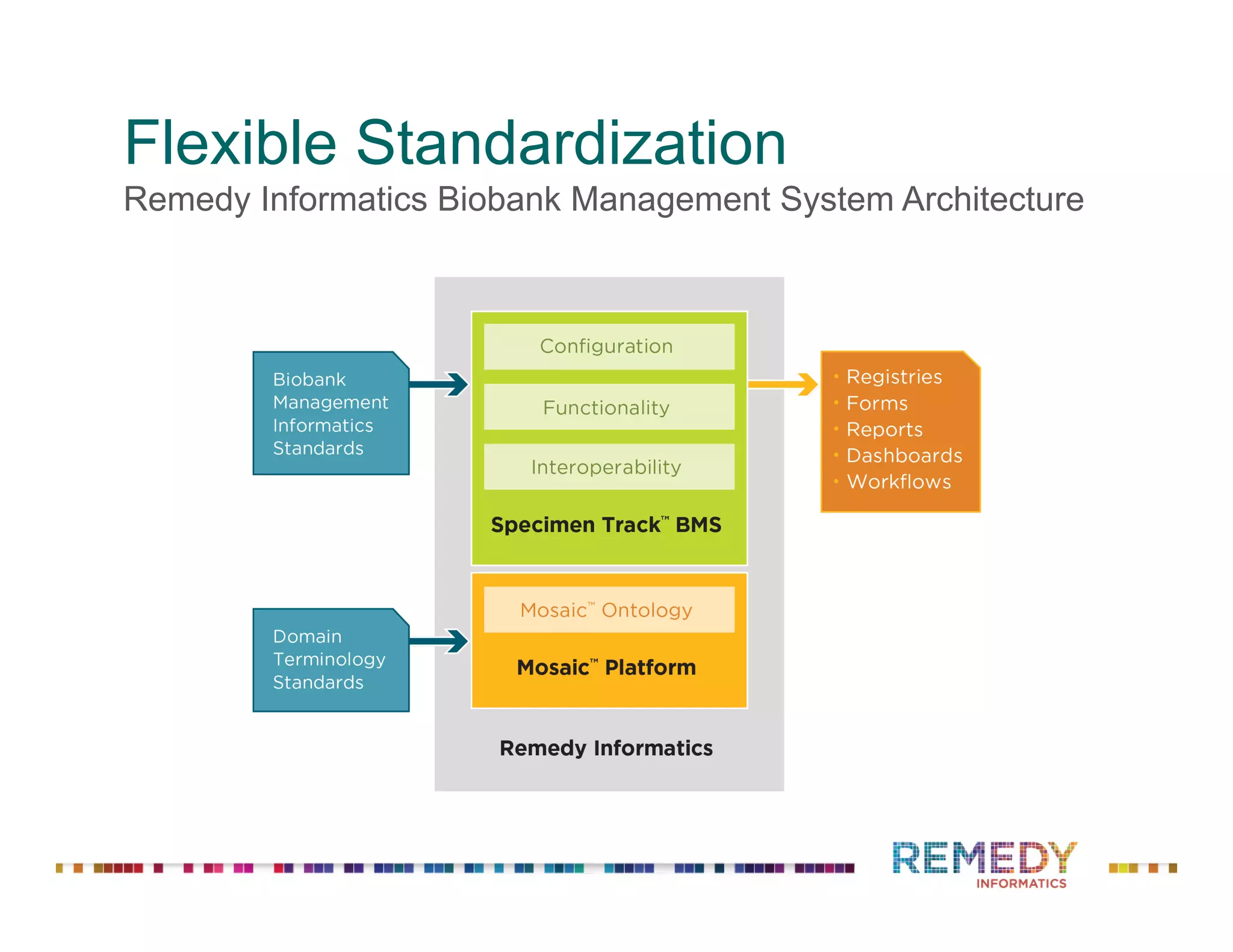
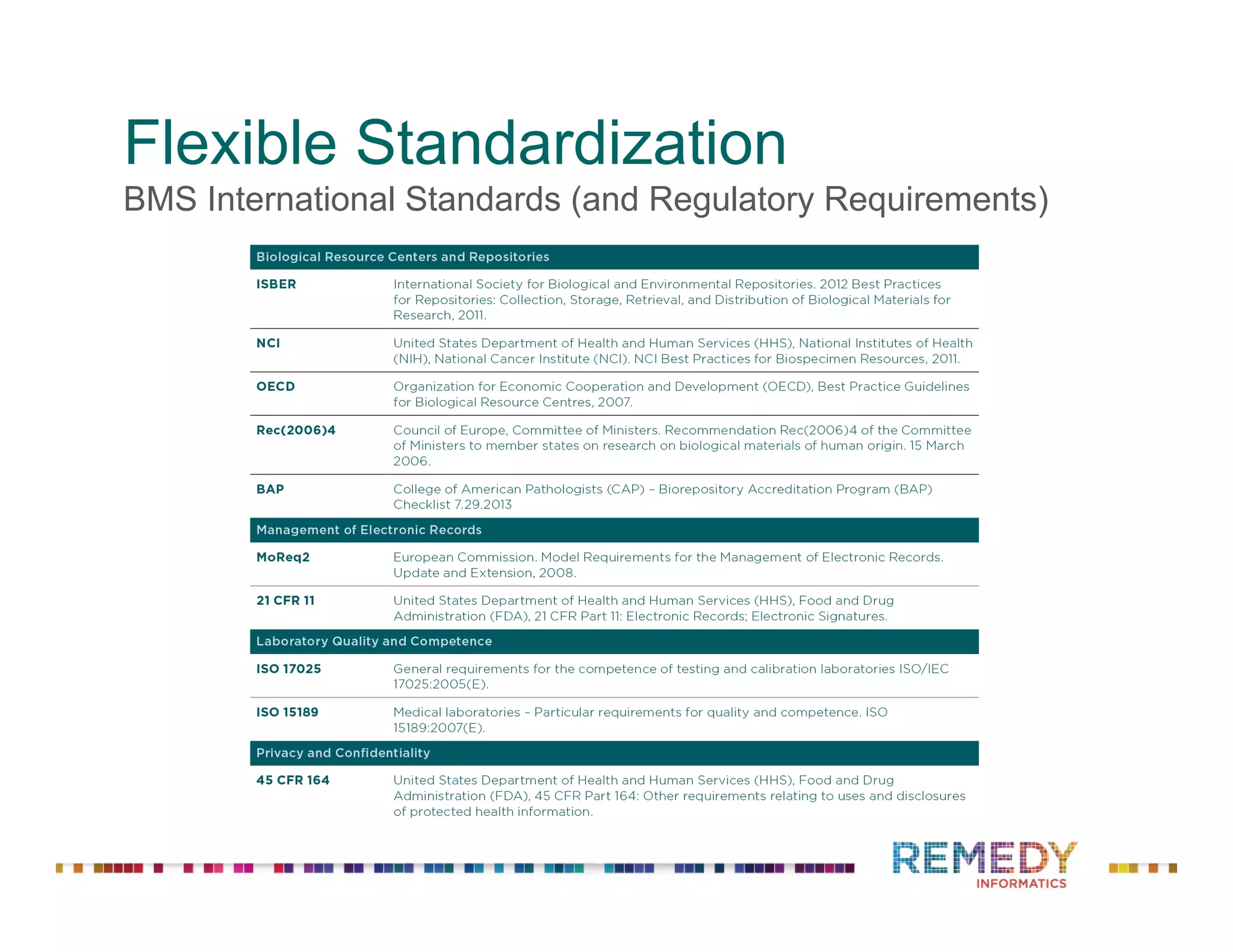
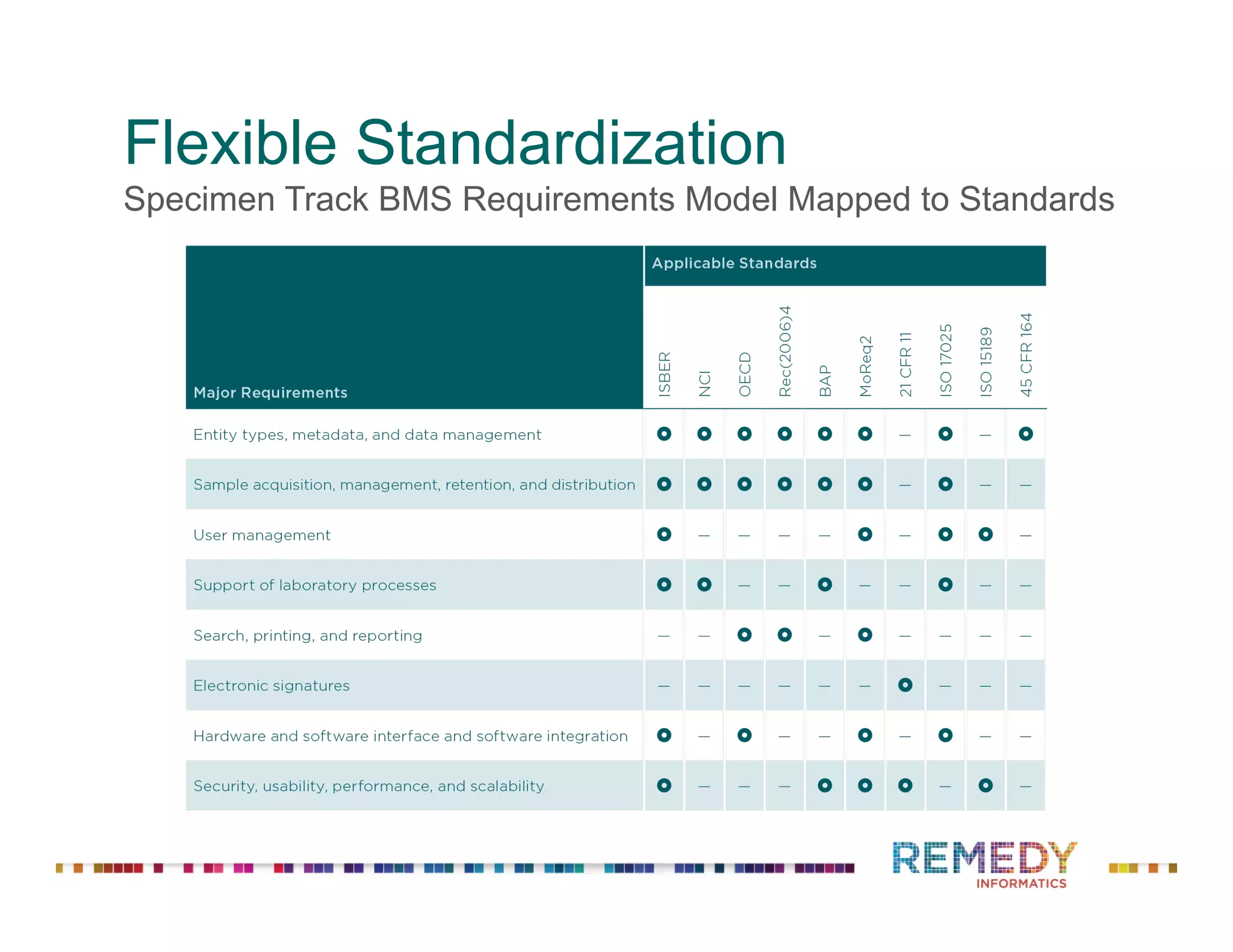
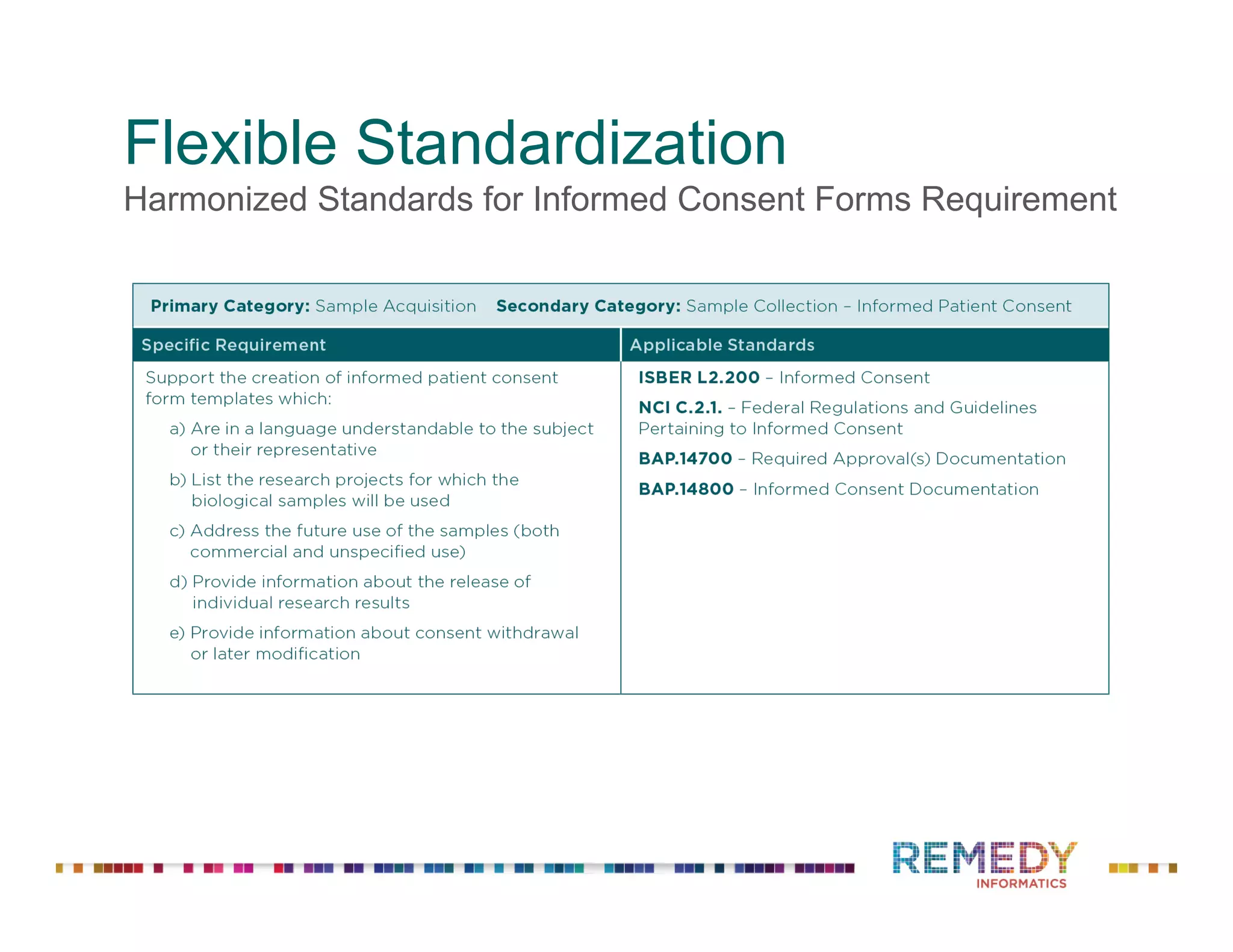
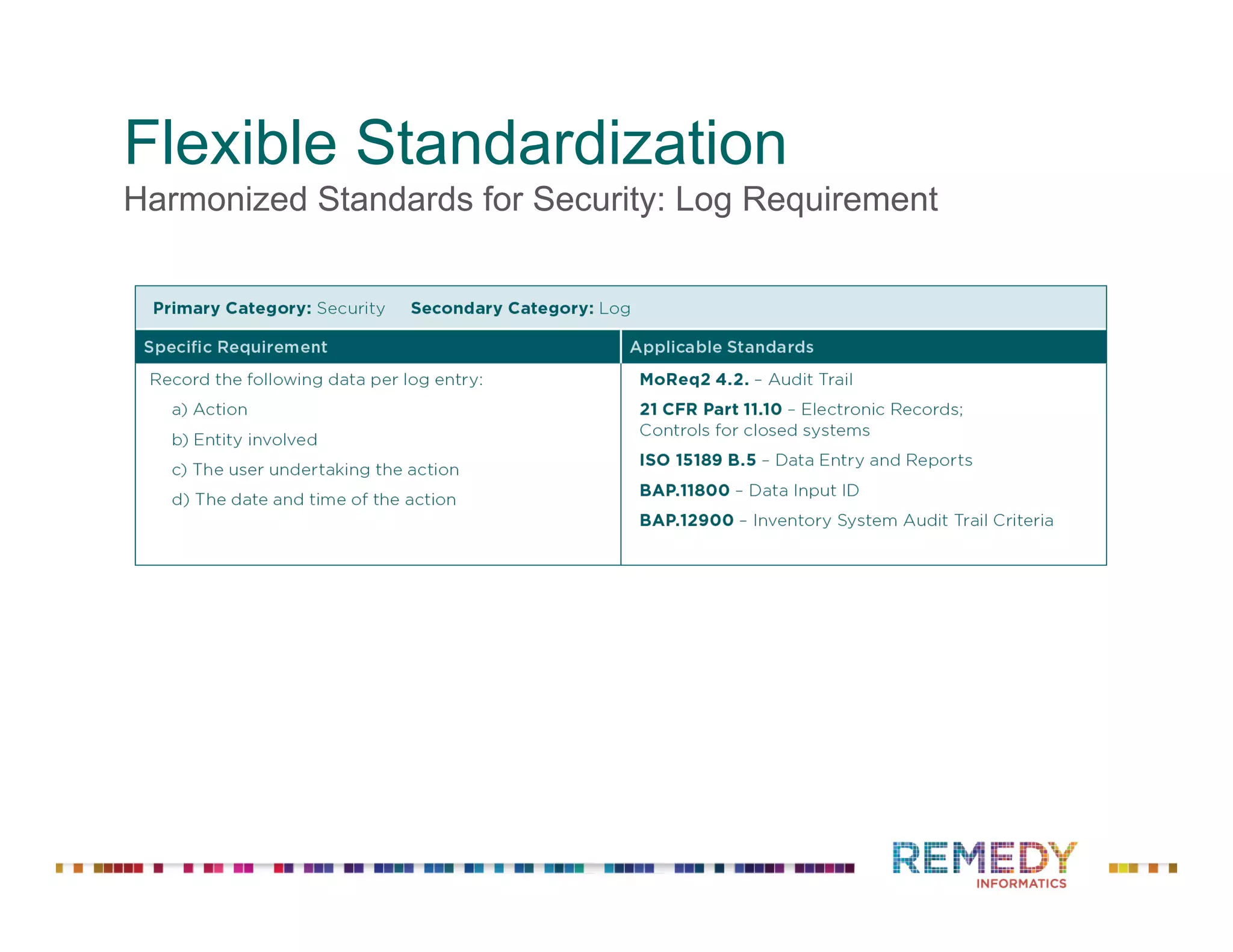
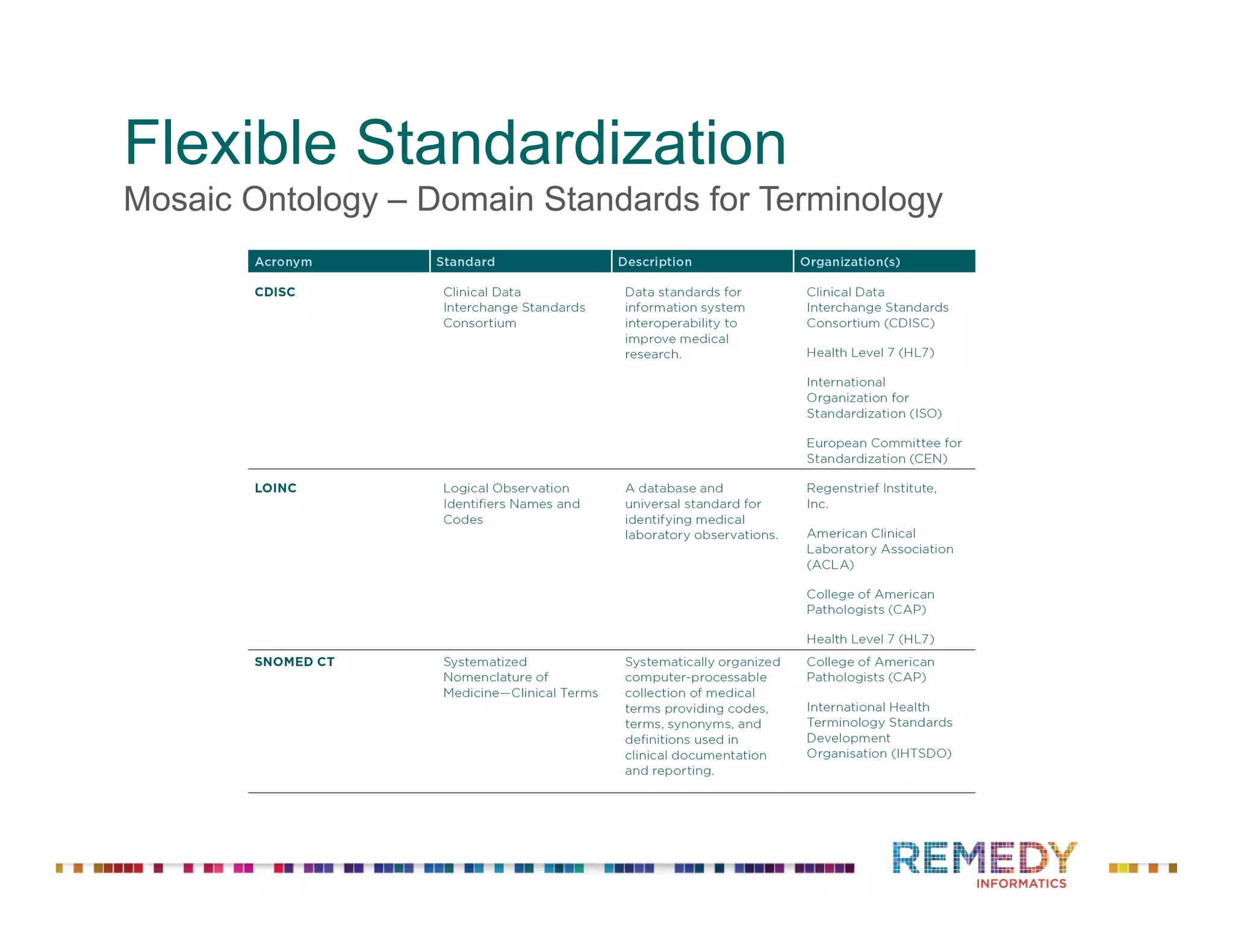
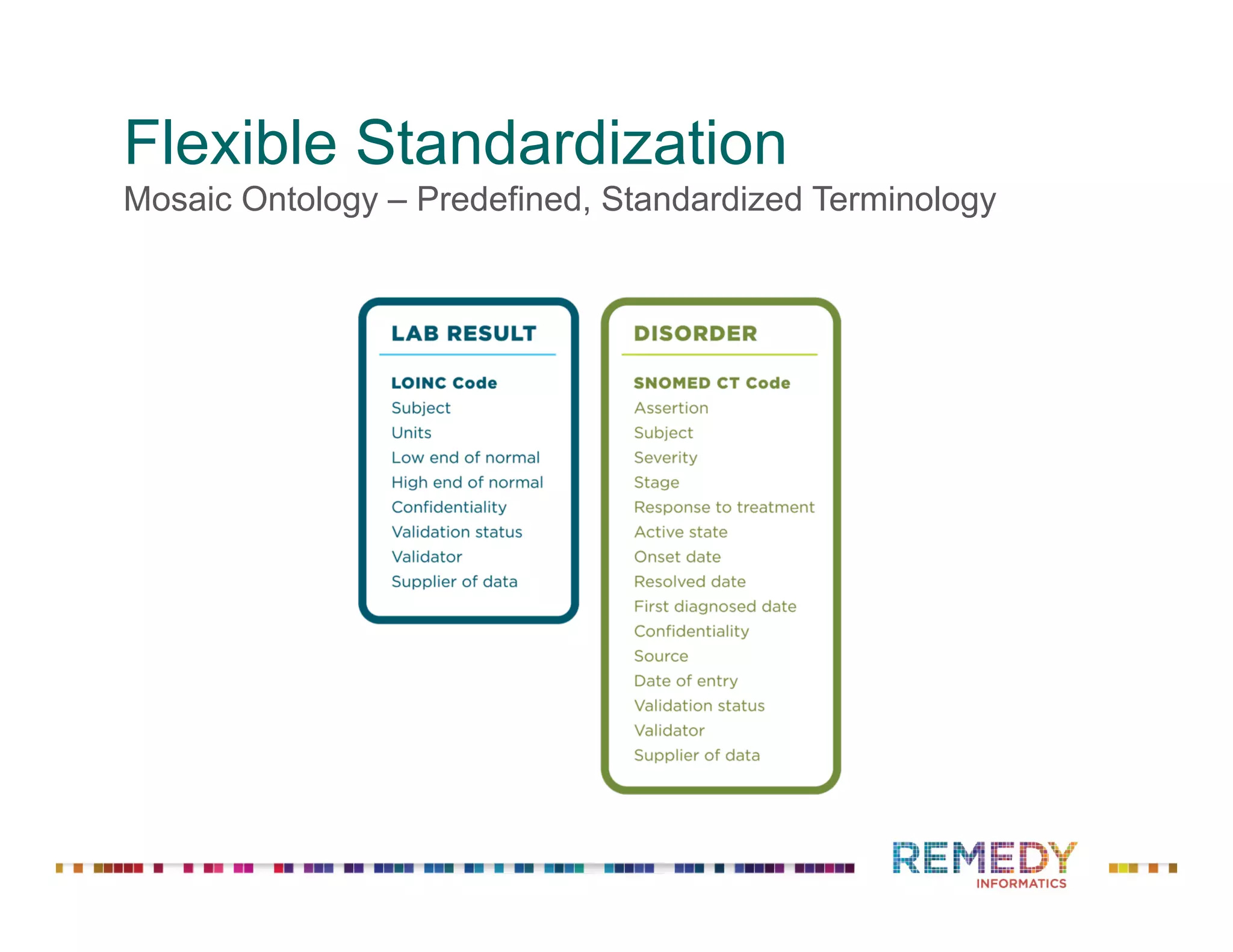
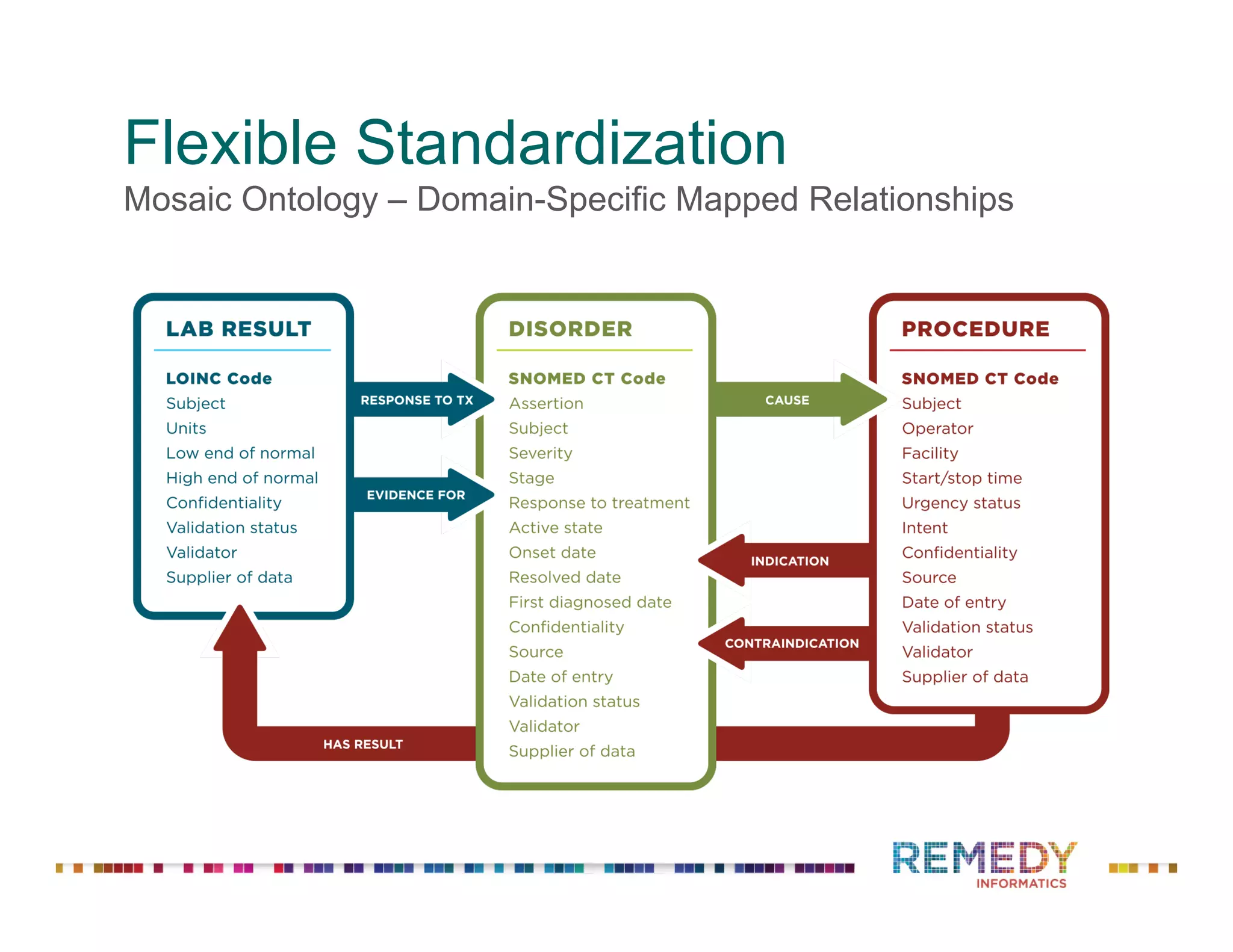
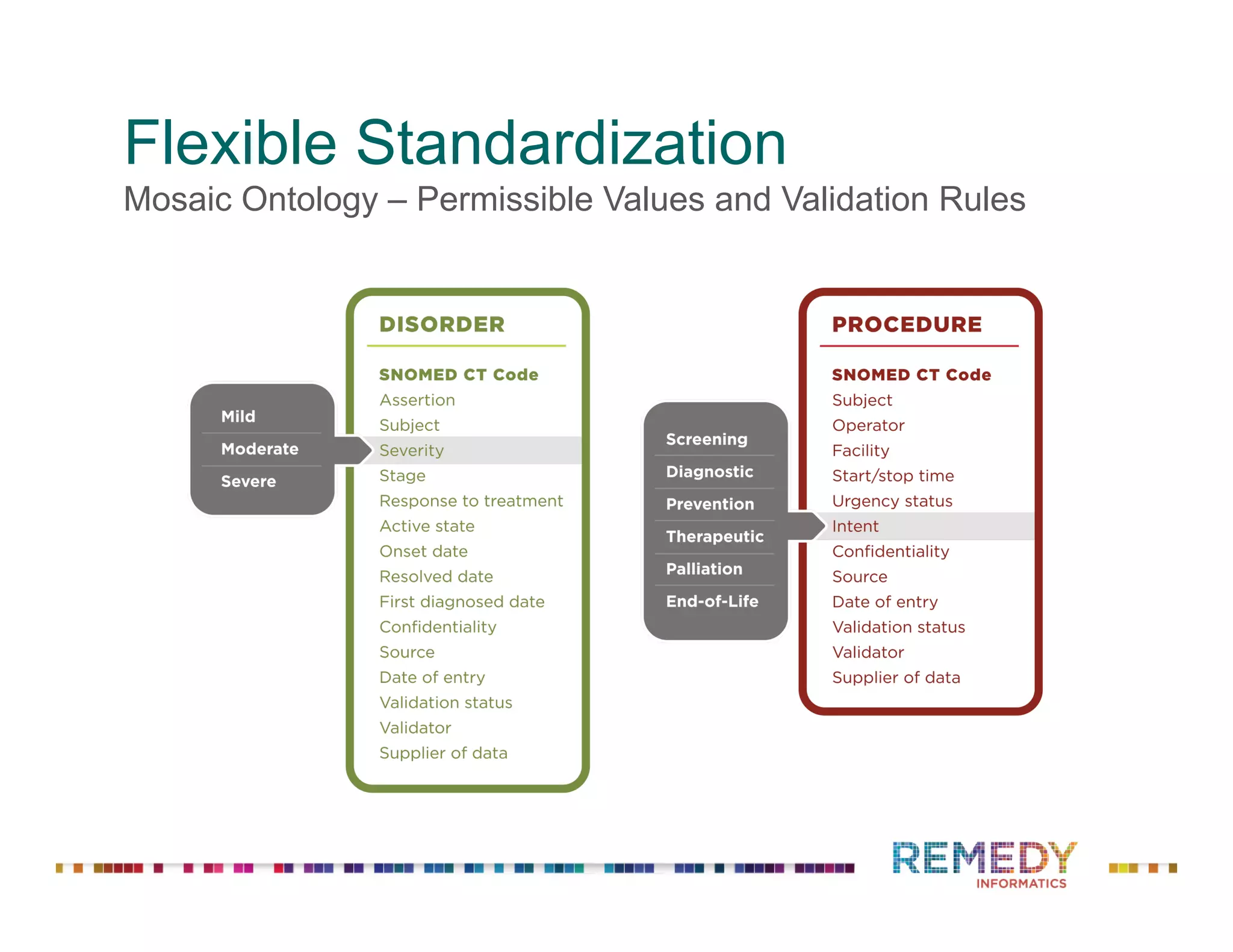
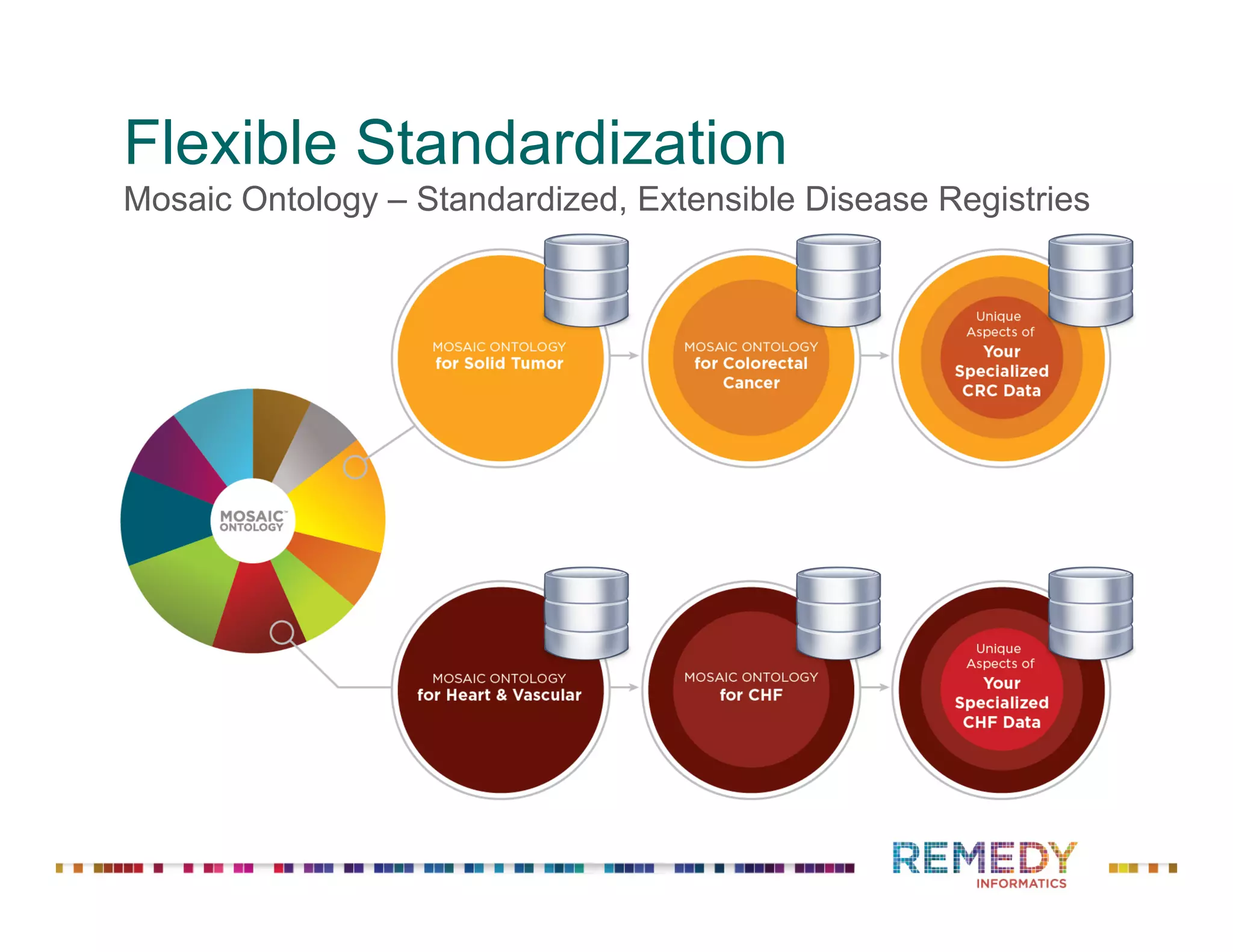
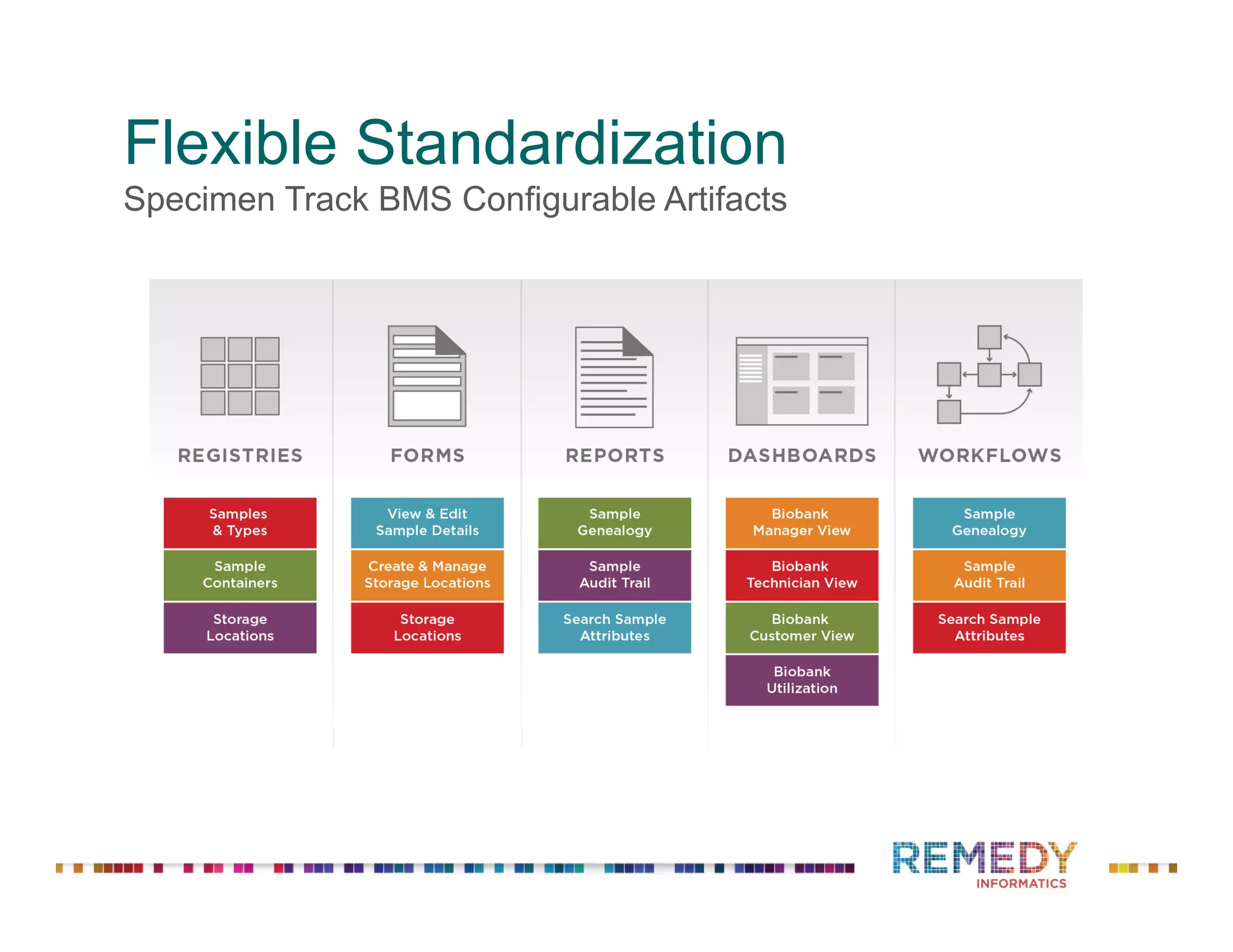
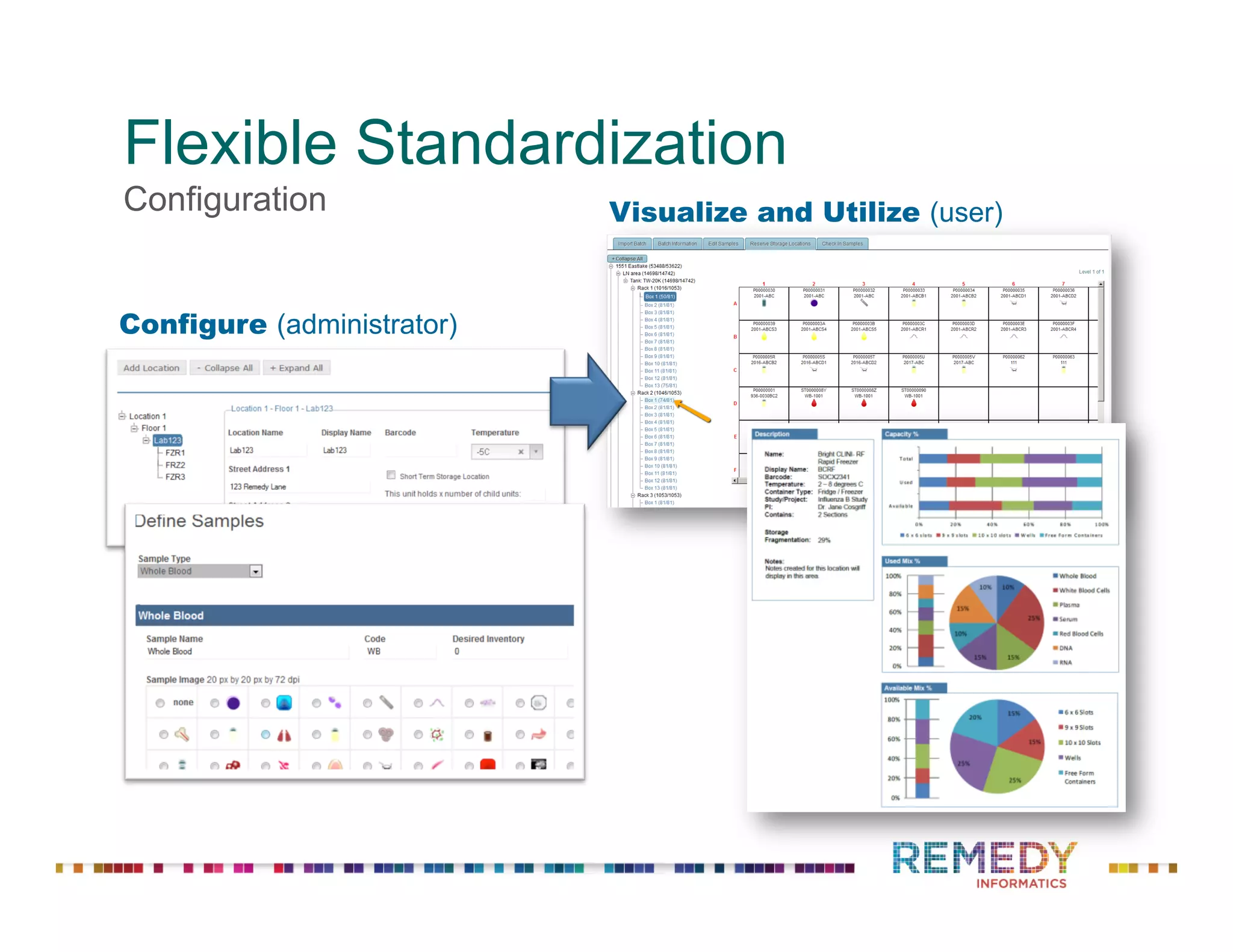
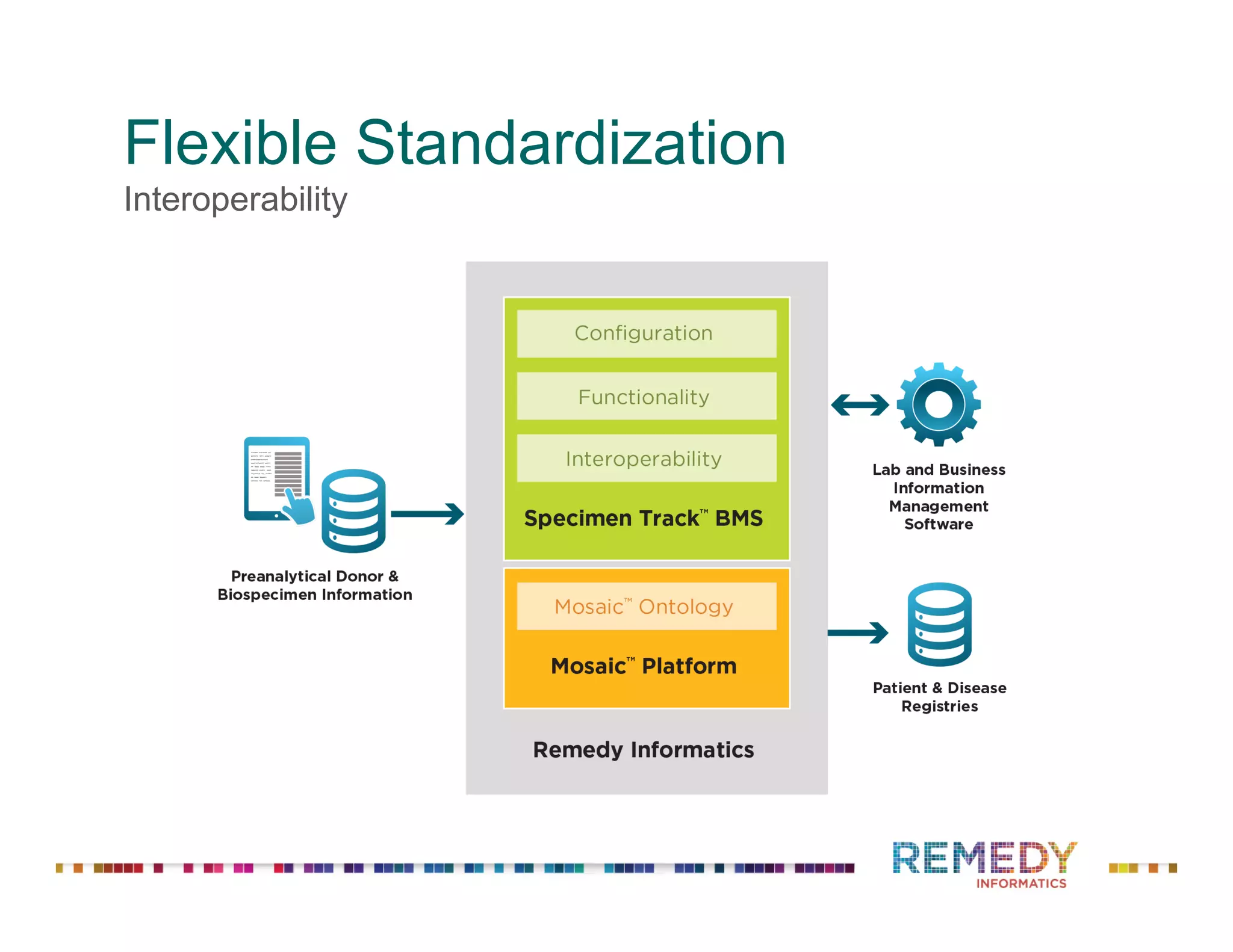
The document discusses a presentation on flexible standardization in biobank management informatics, emphasizing the role of biobanks as active contributors to disease research and therapeutic development. It outlines the features of the Remedy Informatics biobank management system (BMS), which integrates flexibility and standardization across biospecimen and donor information management. Bruce Pharr, the author, presents the product roadmap and insights gained while working with Remedy Informatics to enhance biobank operations.