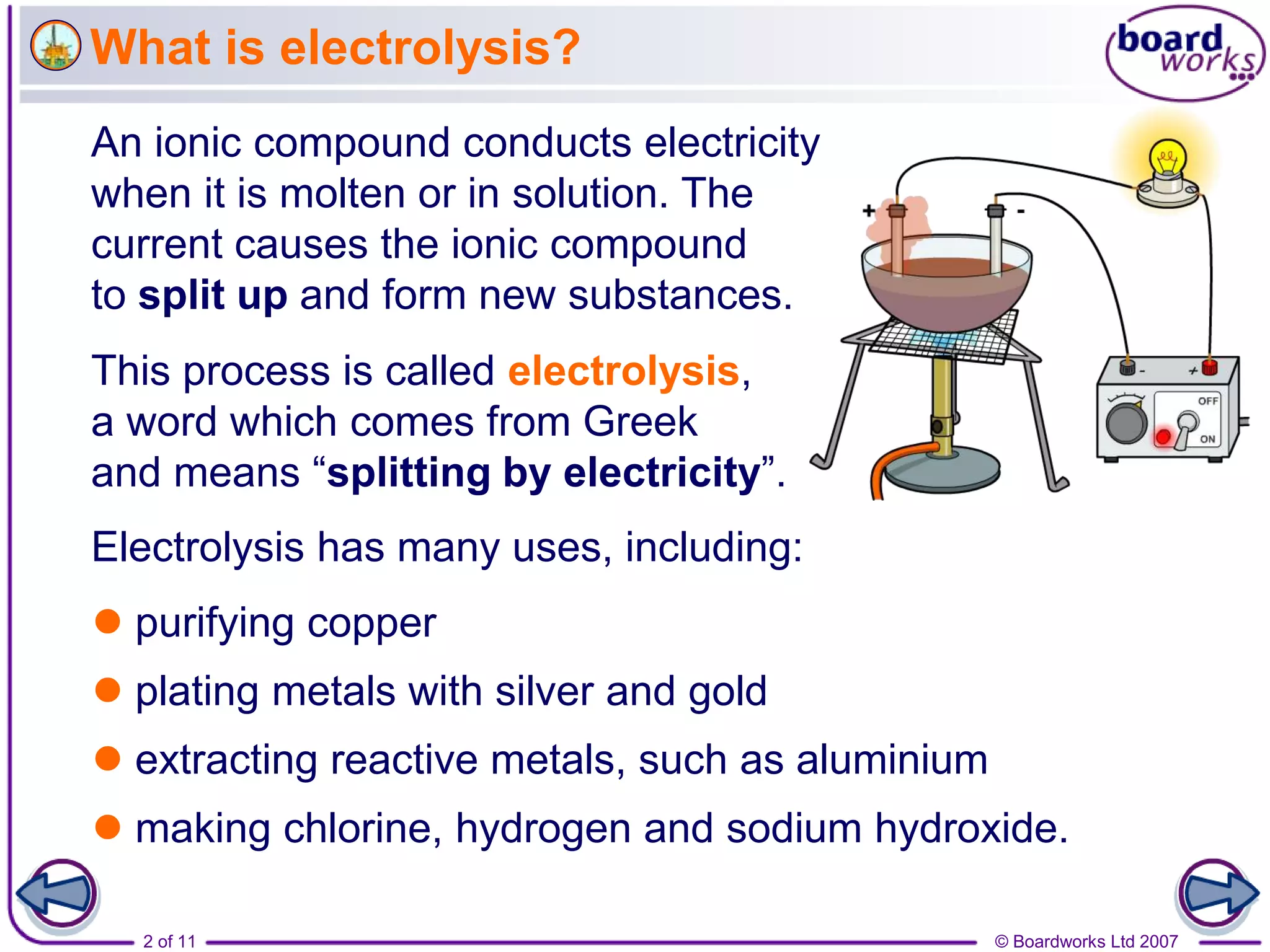
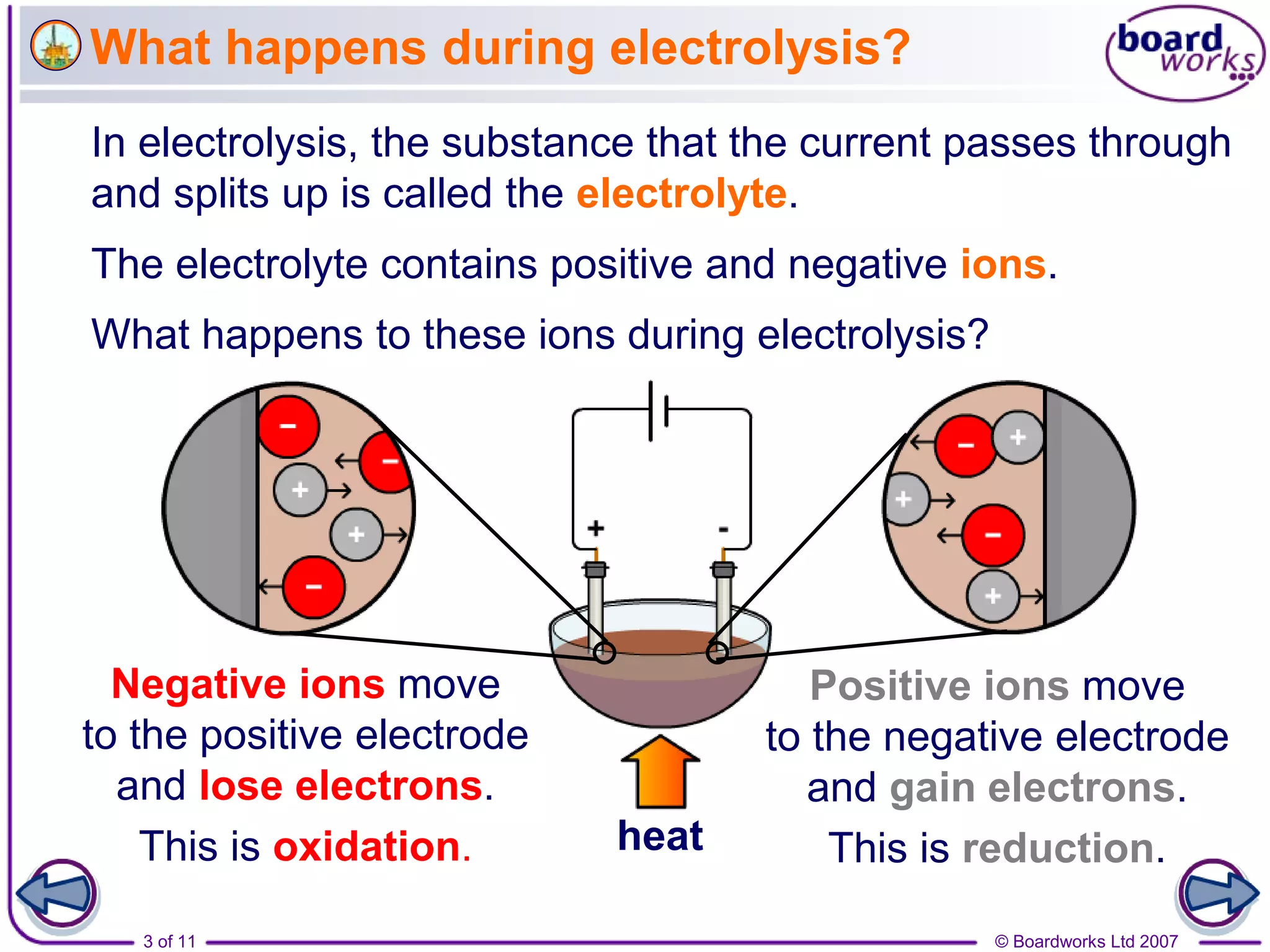
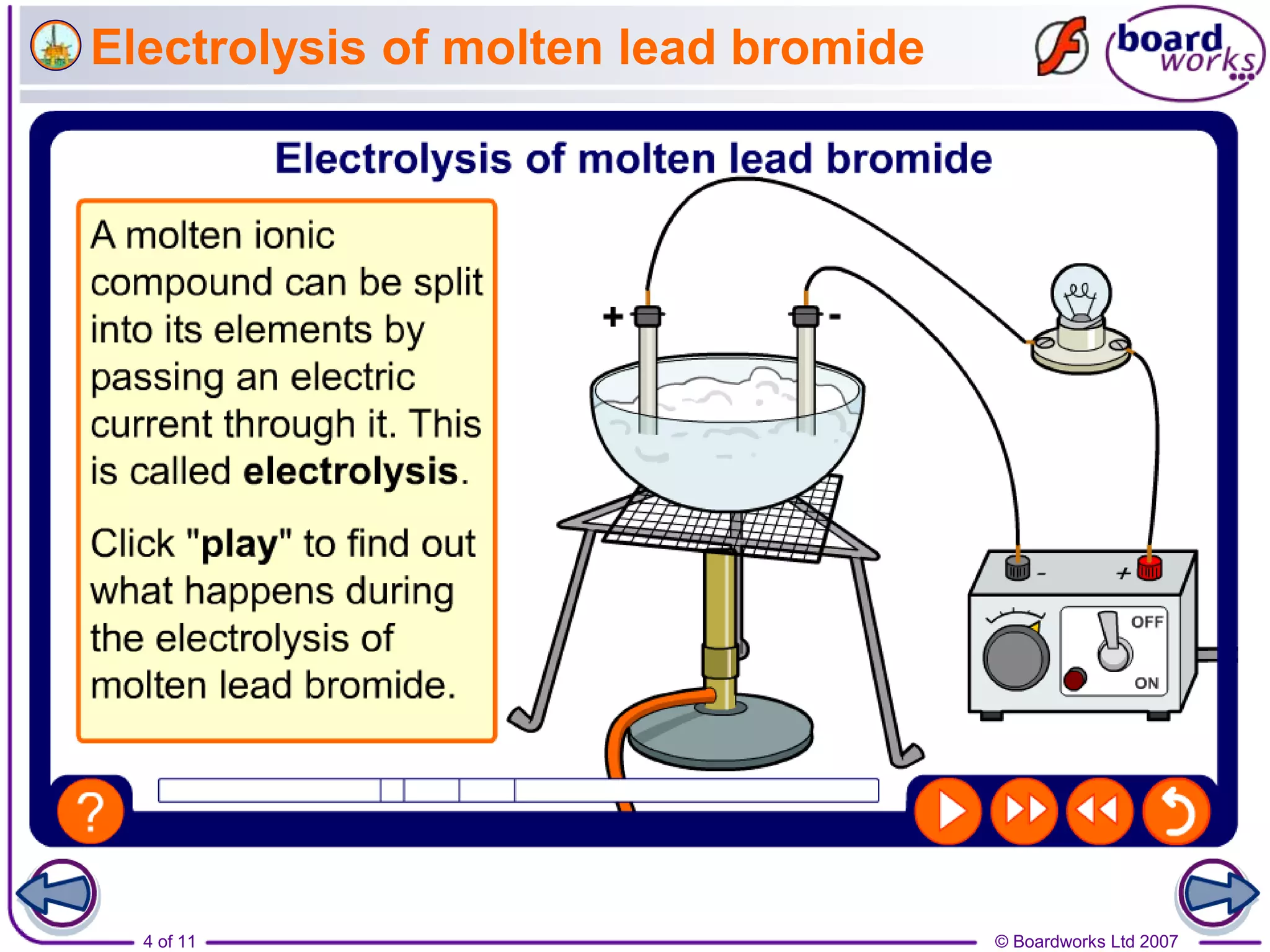
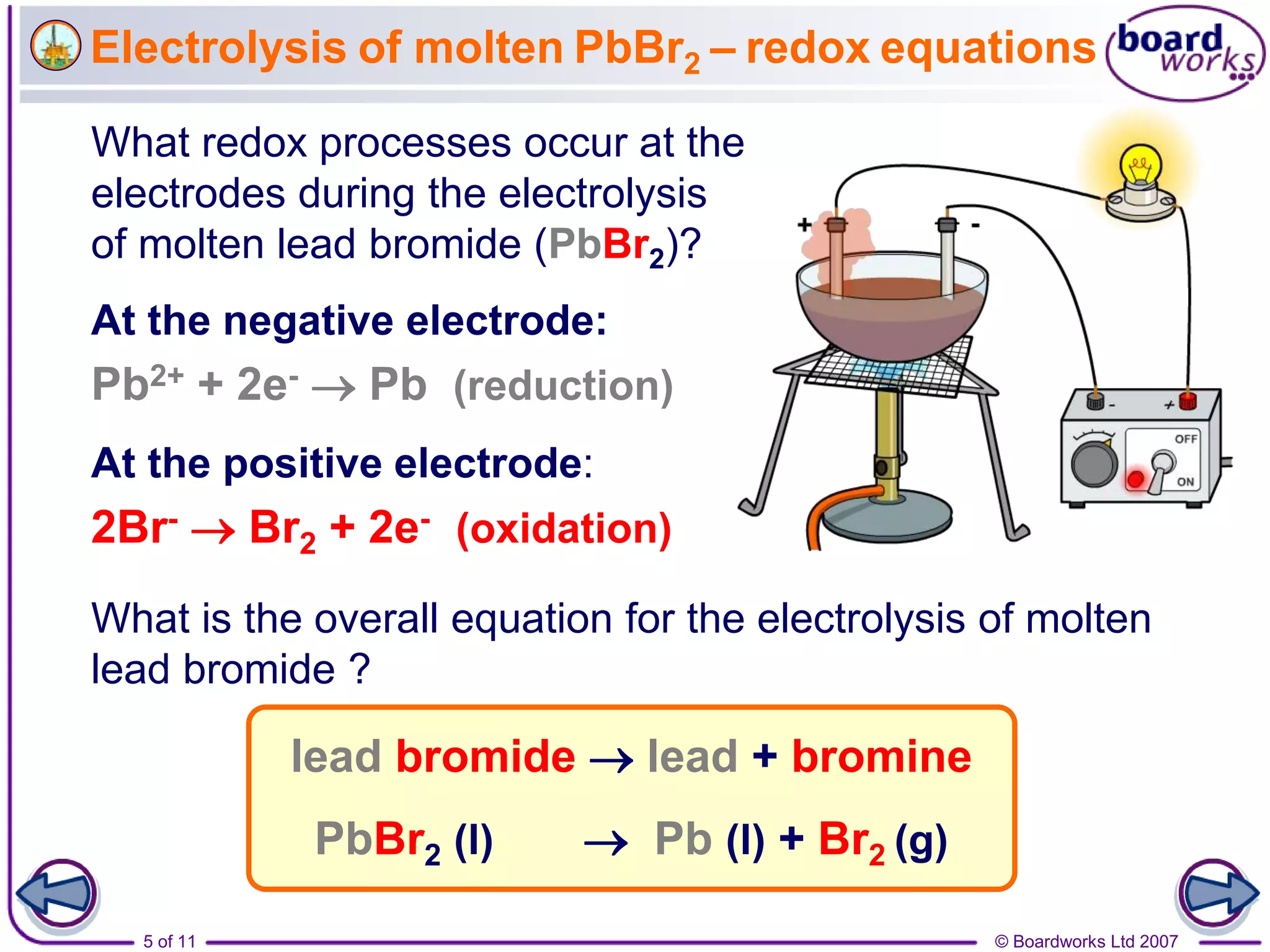
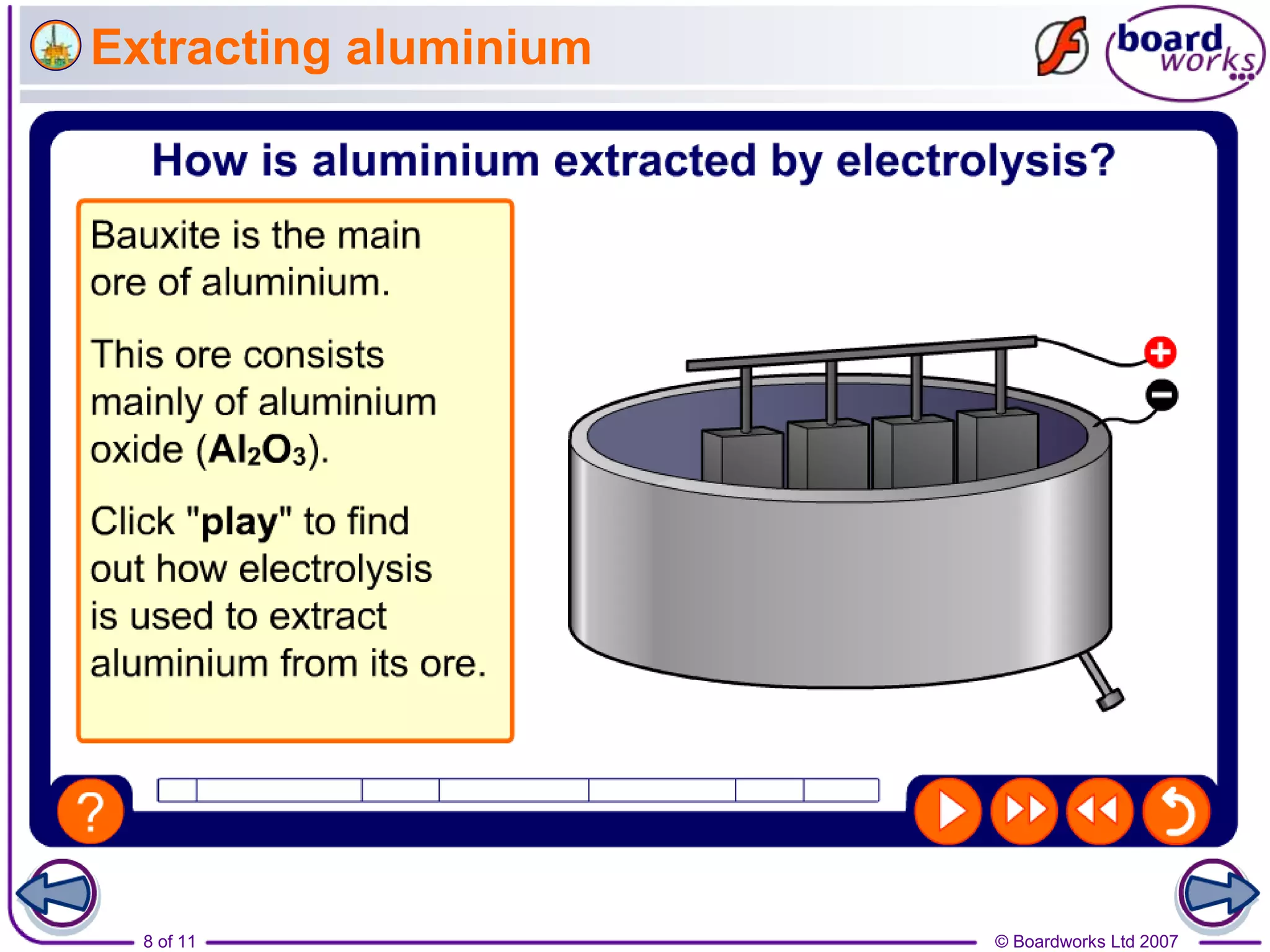
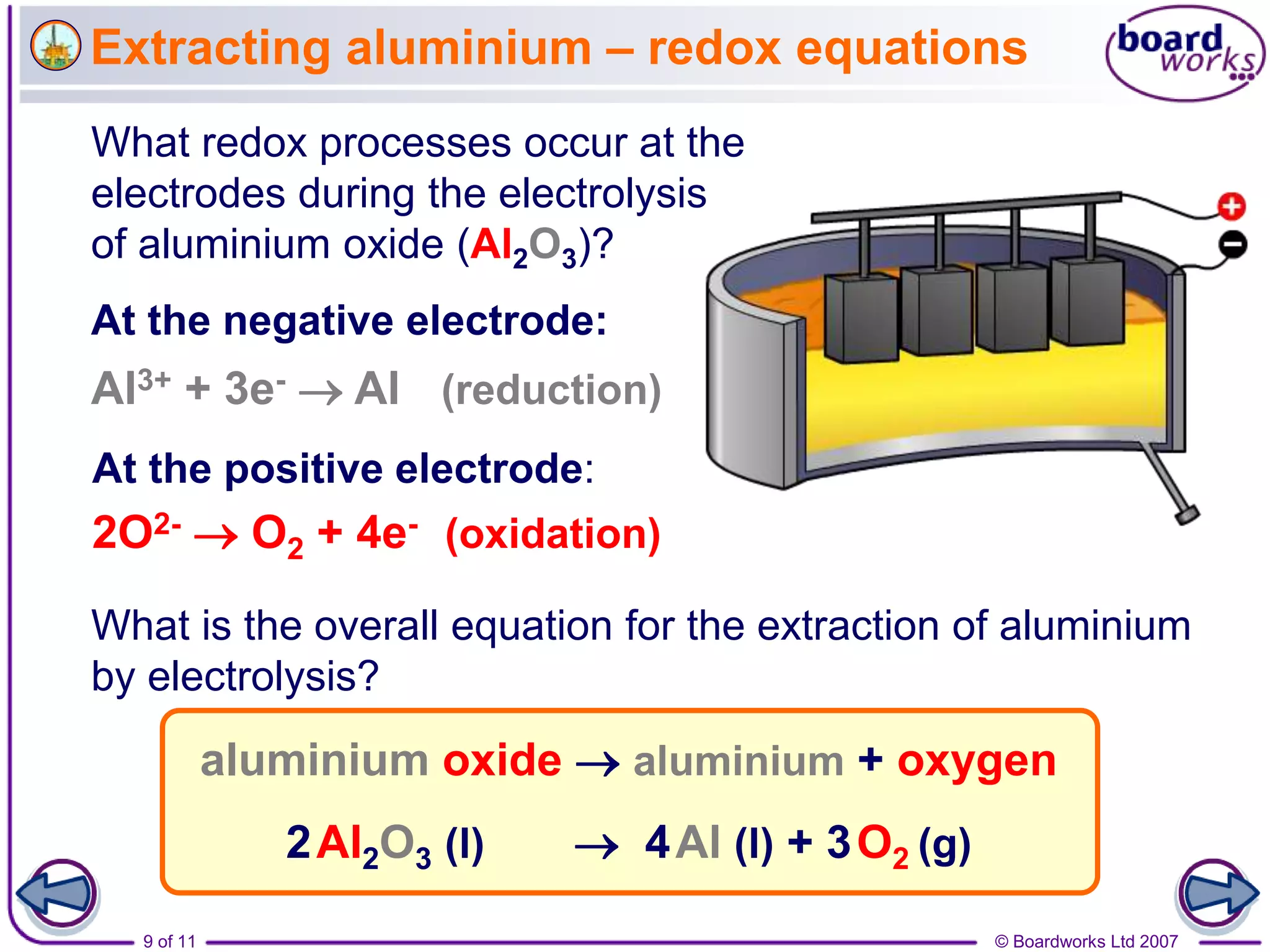
Electrolysis is a process where an electric current is passed through an ionic substance, causing the substance to decompose. During electrolysis, the substance being electrolyzed (called the electrolyte) contains positive and negative ions. Positive ions move to the negative electrode where they gain electrons through reduction reactions. Negative ions move to the positive electrode where they lose electrons through oxidation reactions. This process is used industrially for processes like refining metals and producing chemicals.