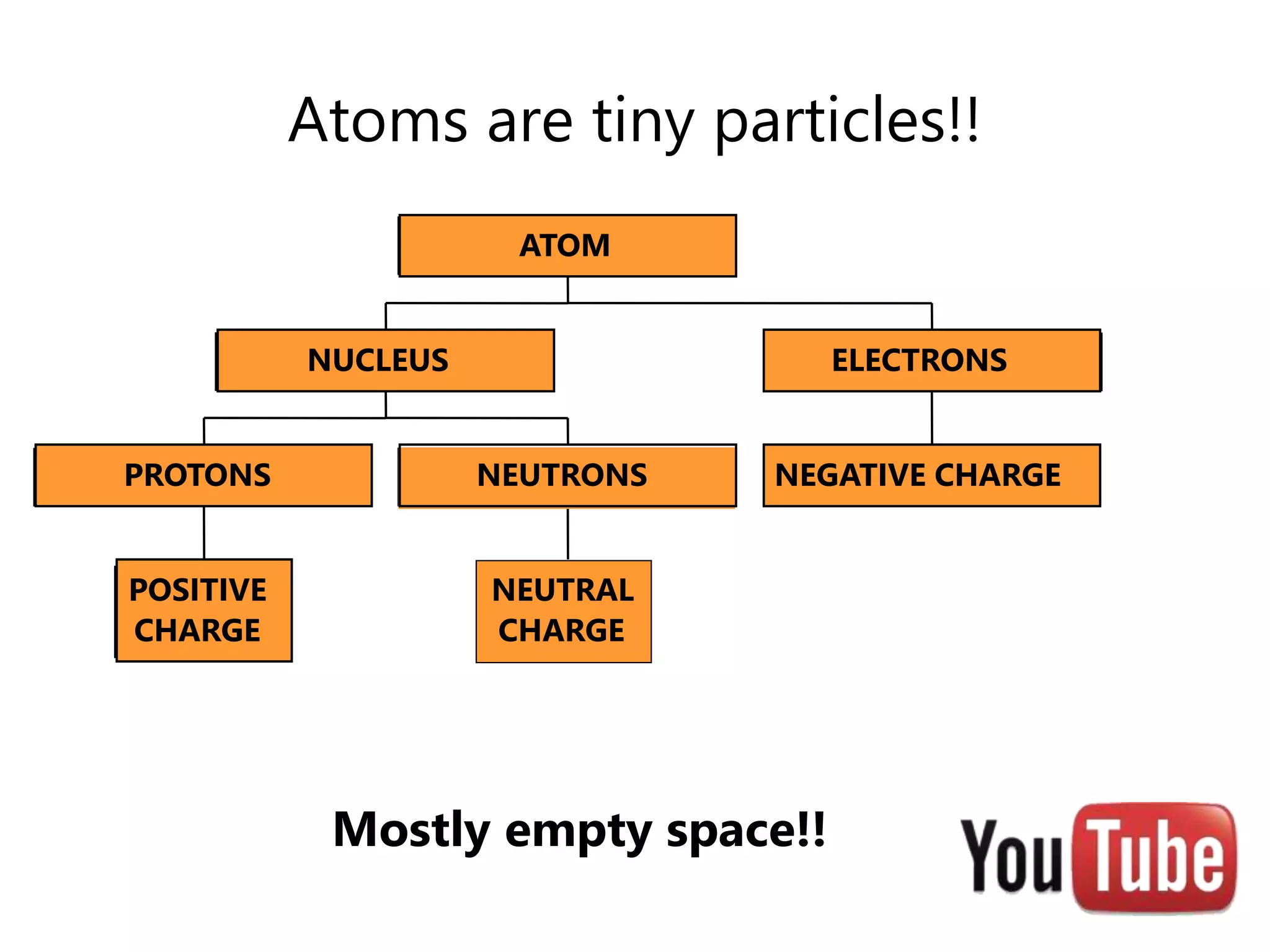
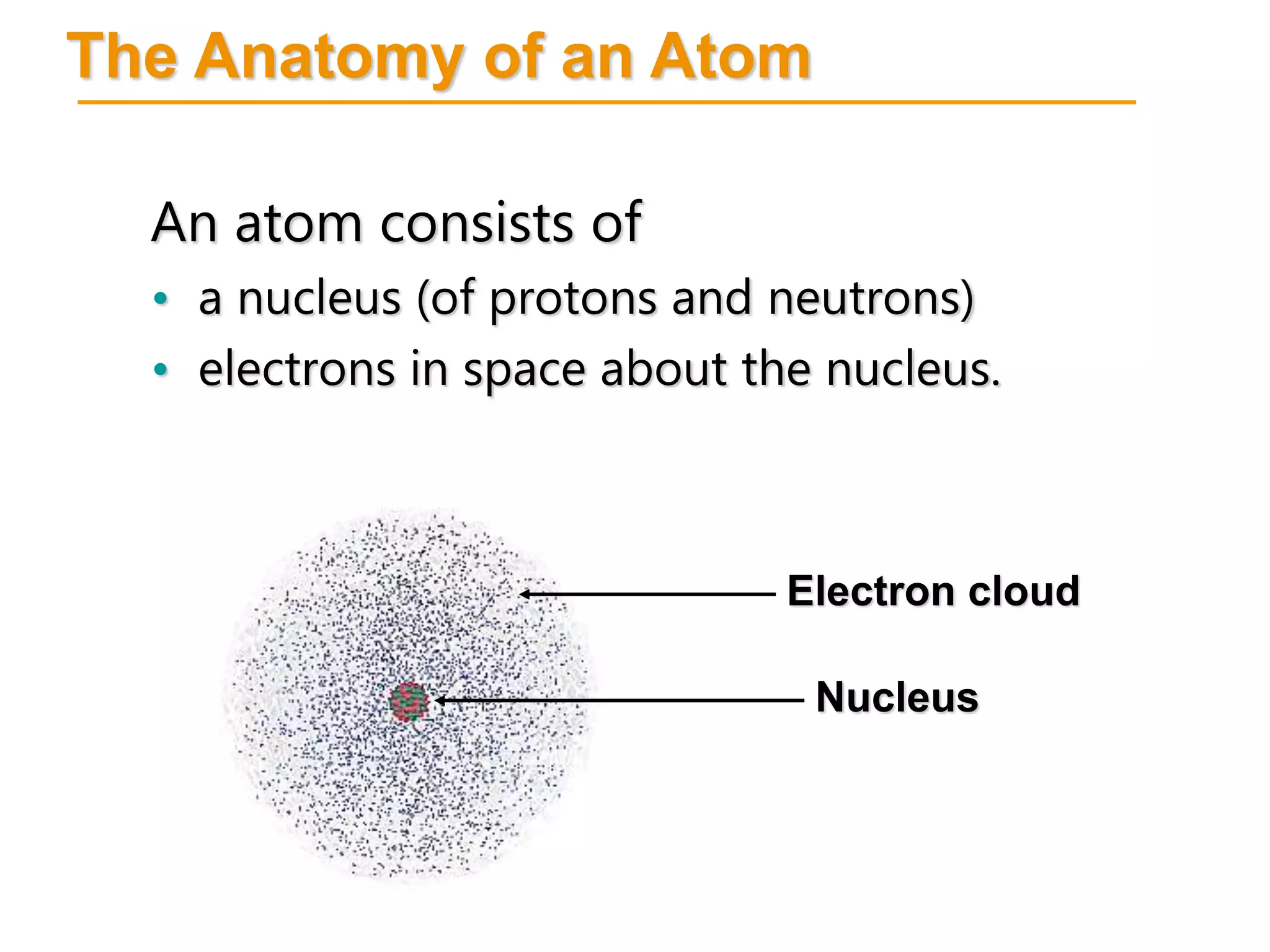
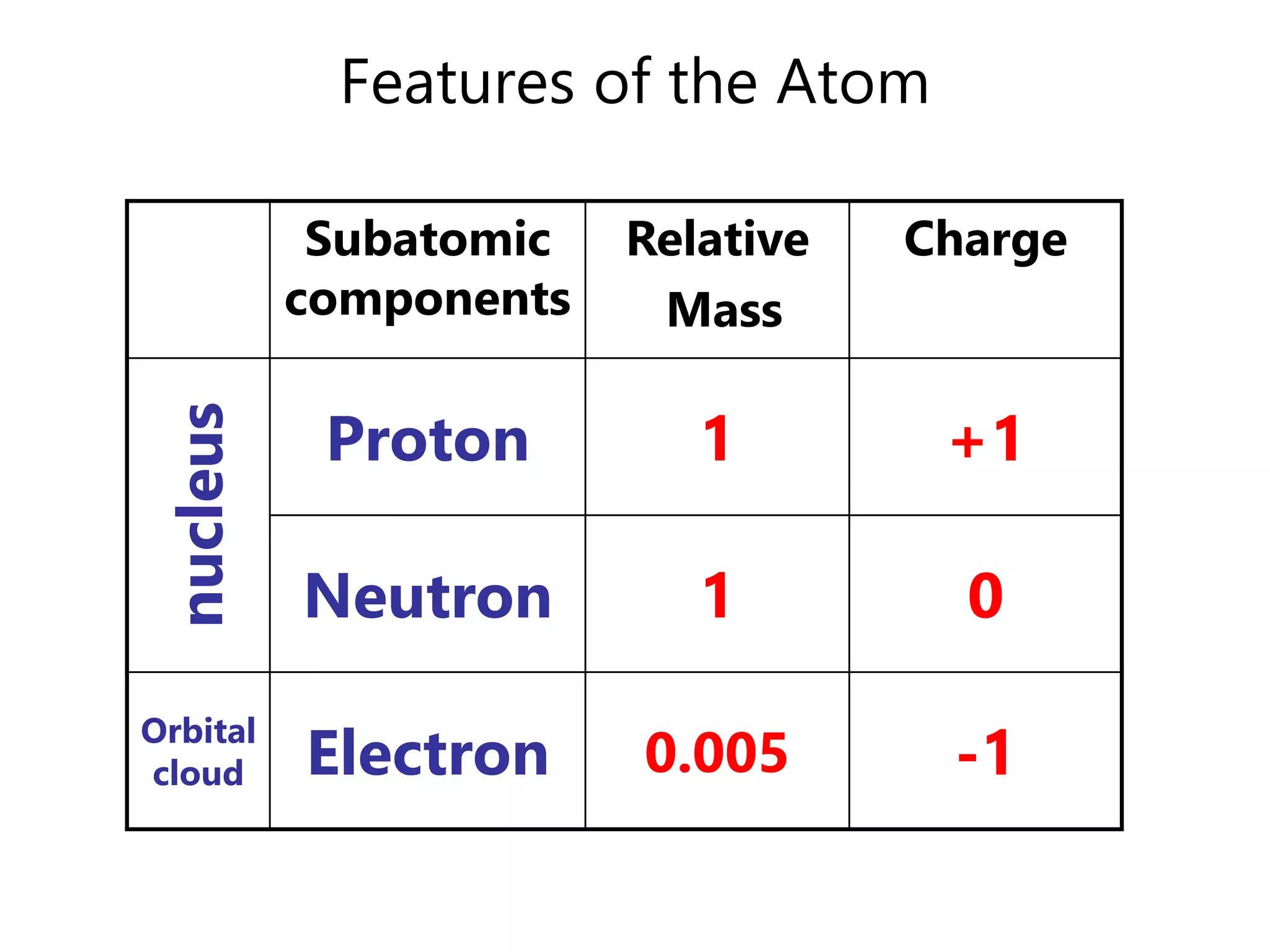
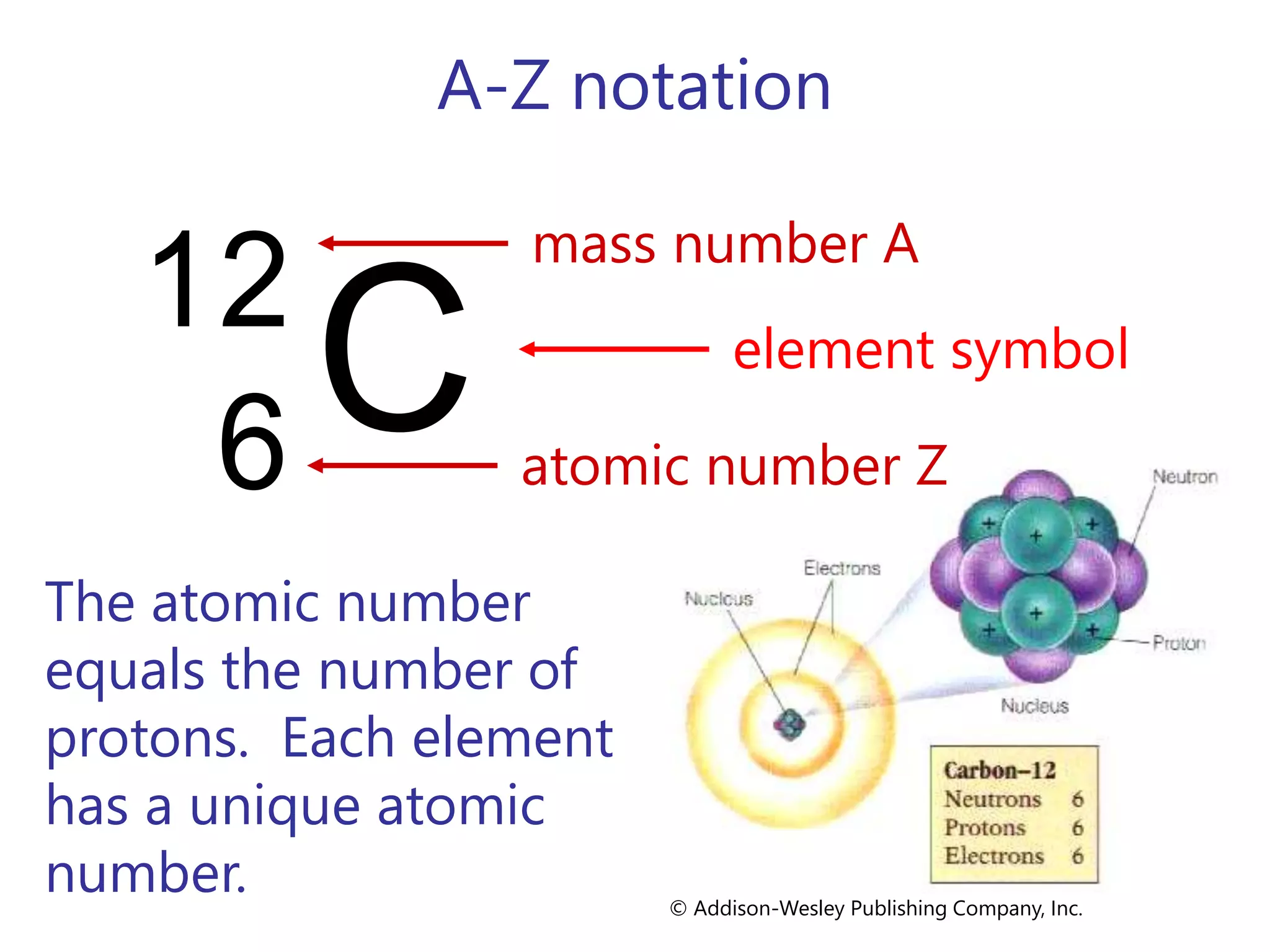
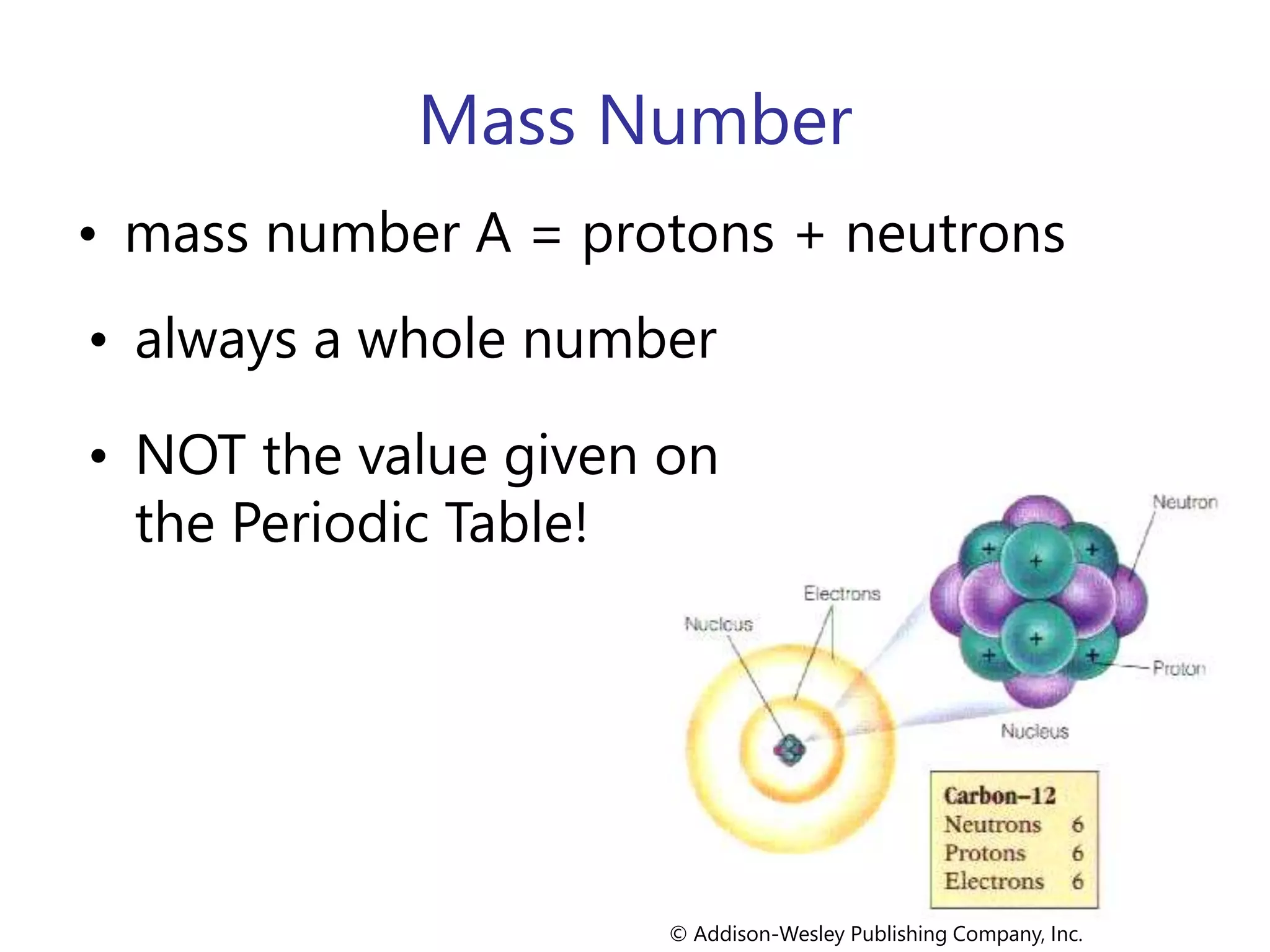
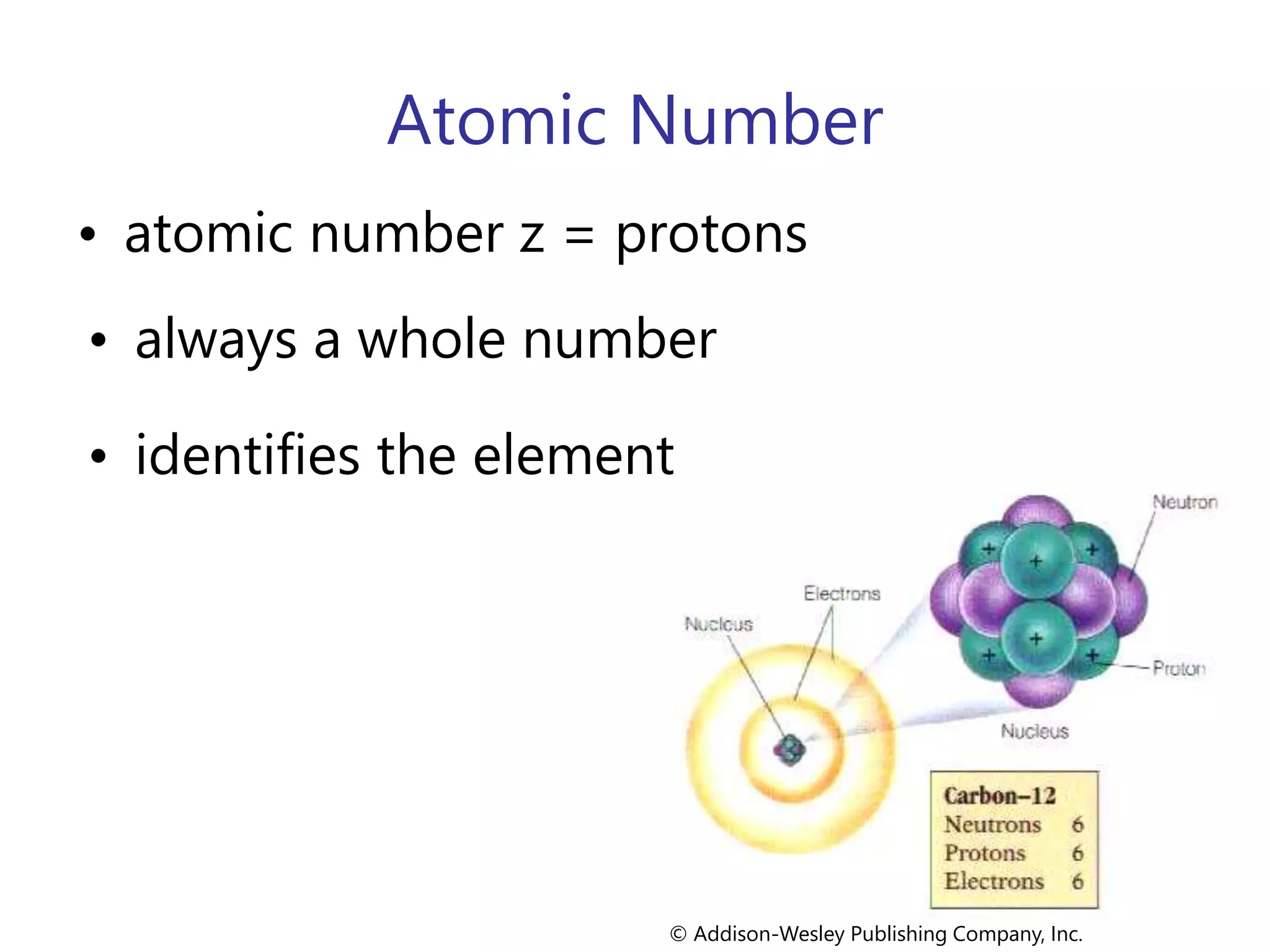
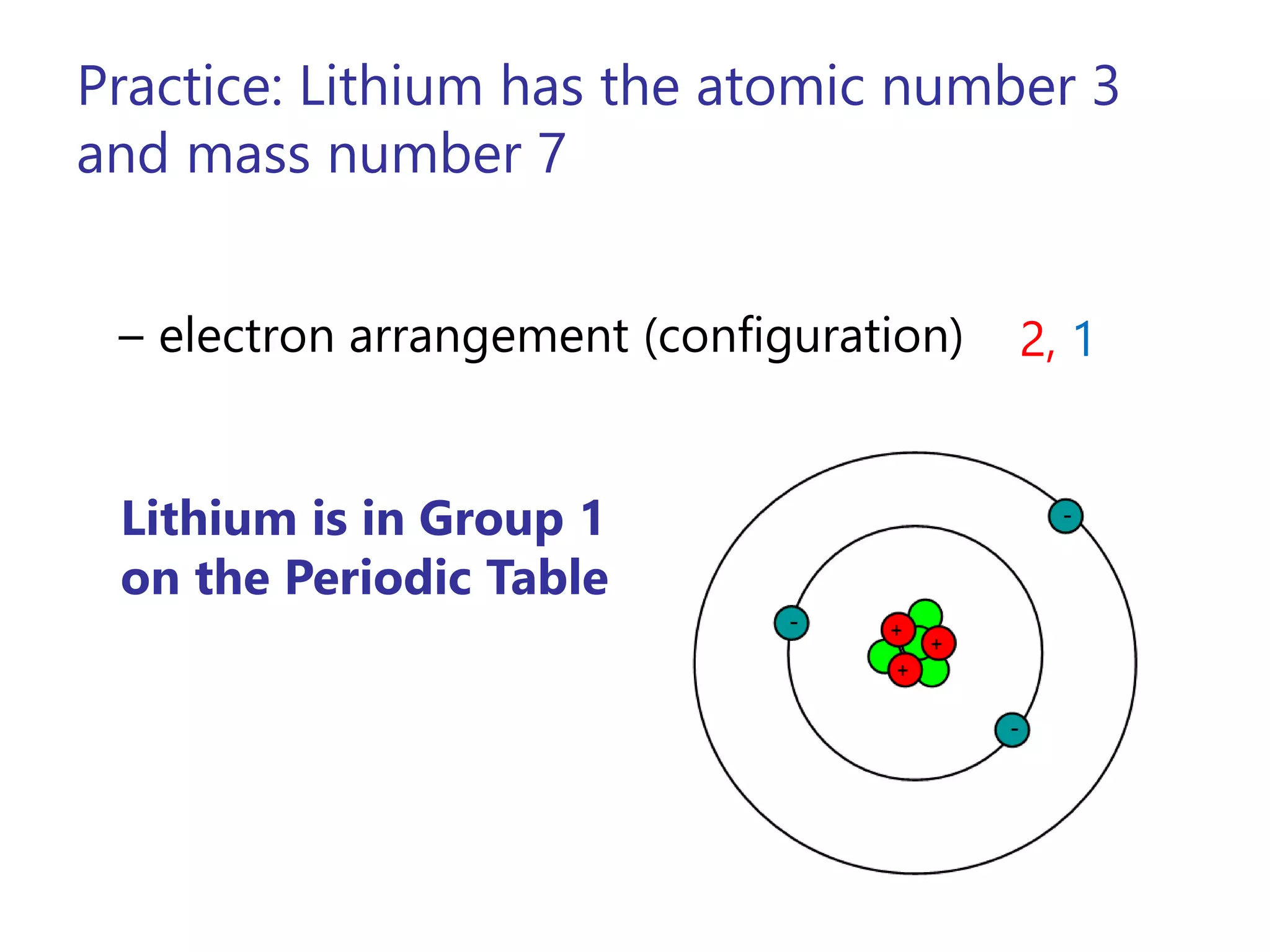
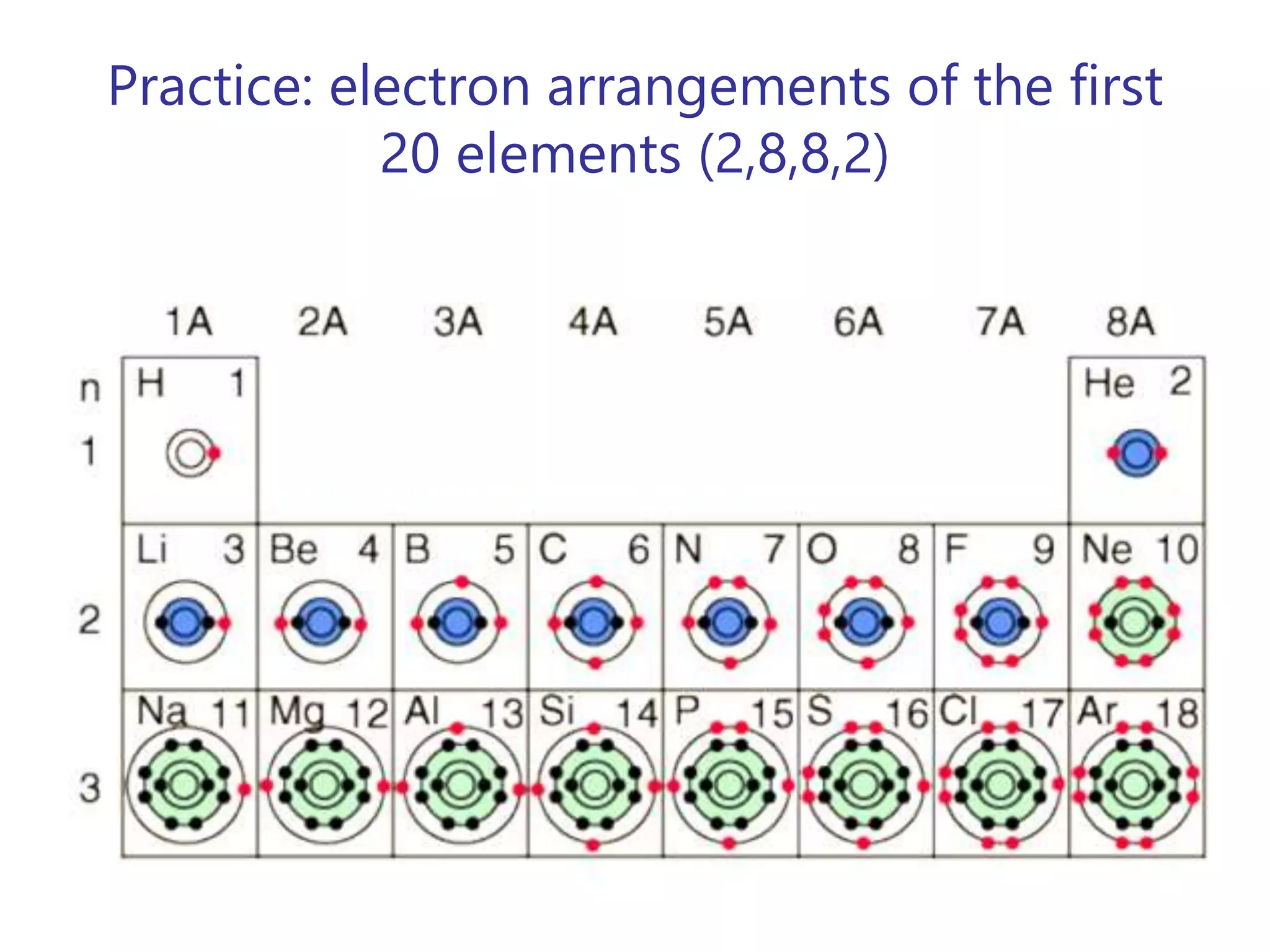
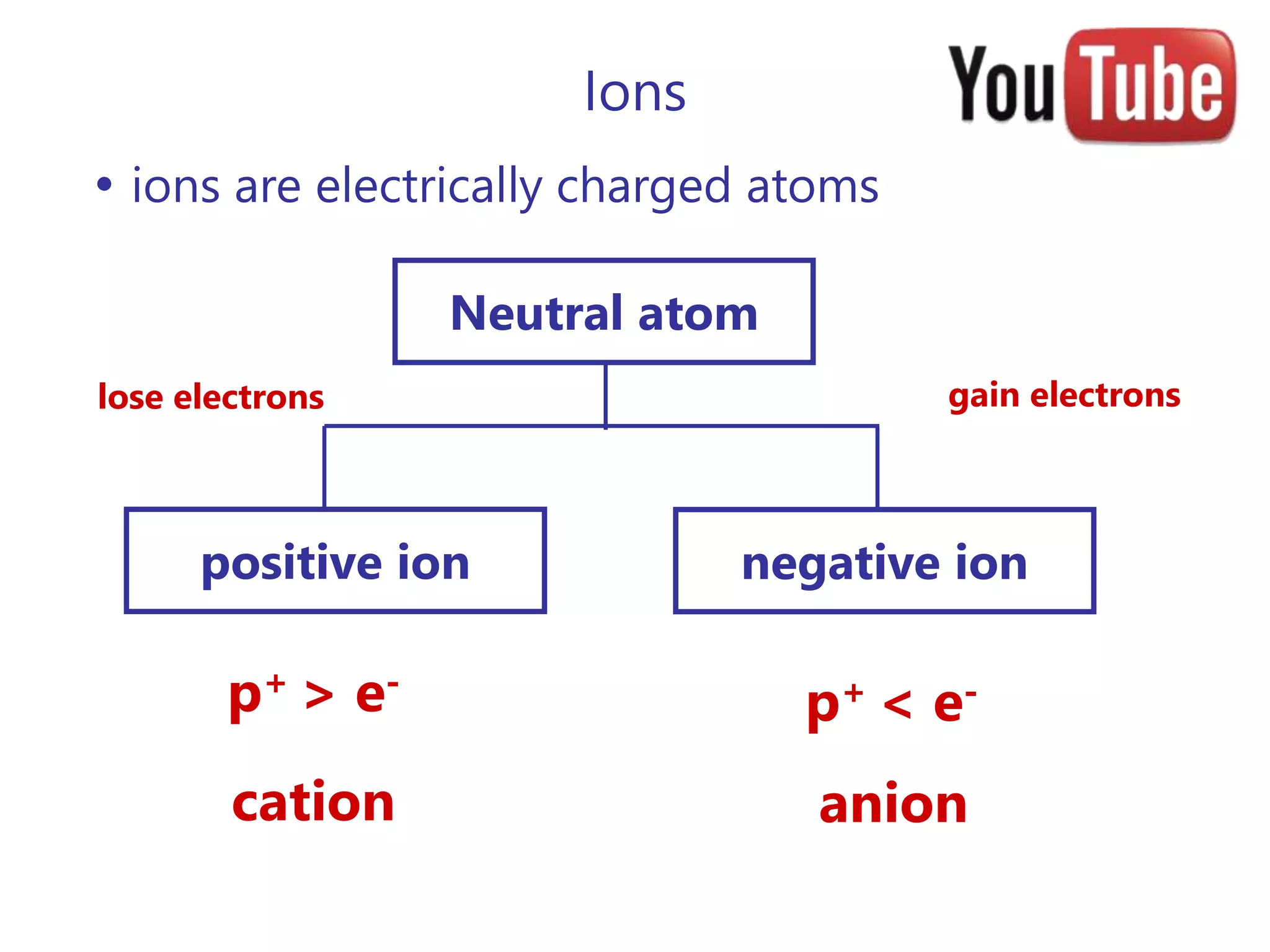
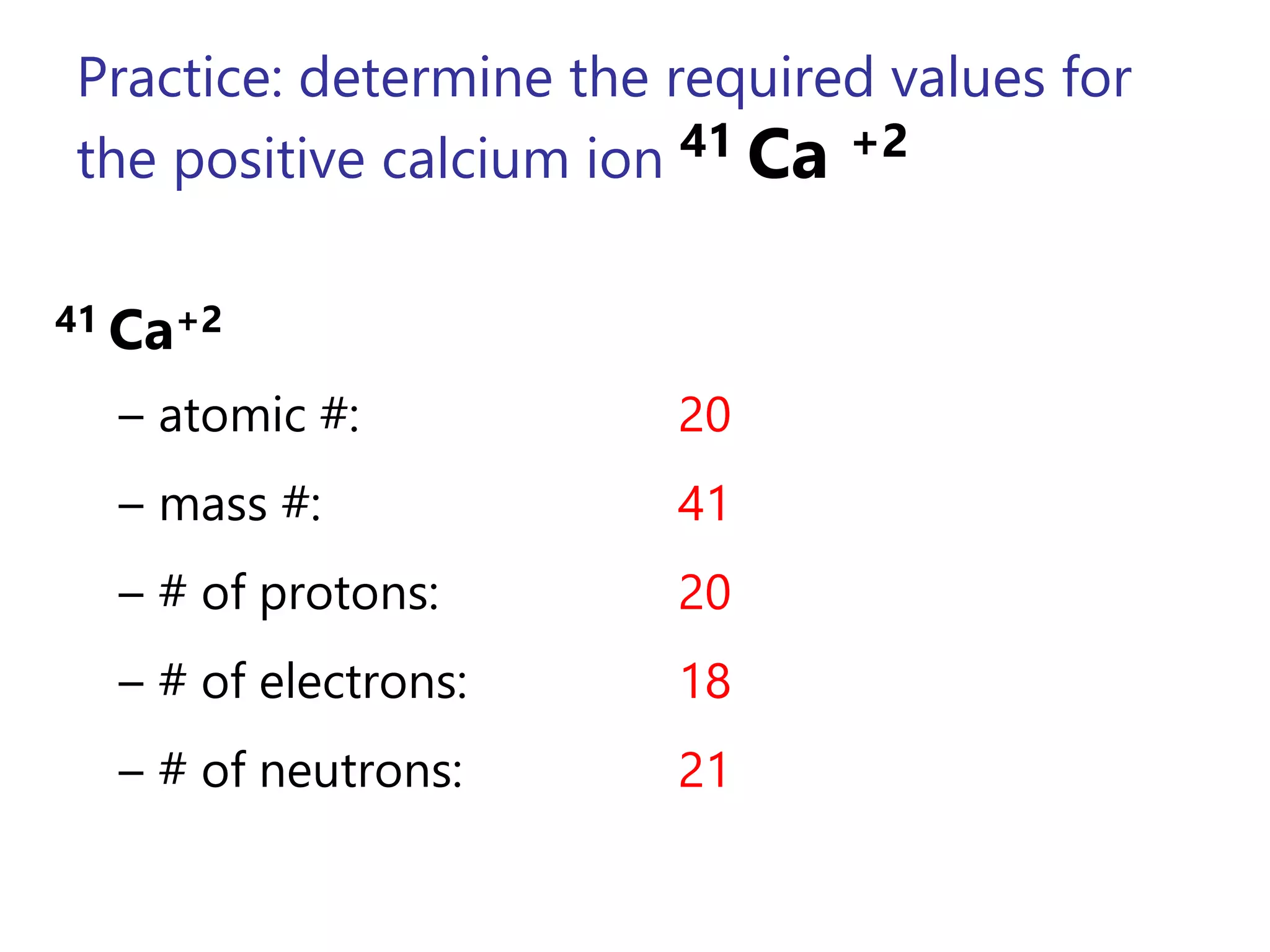
- Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in orbitals.
- The atomic number is the number of protons, which identifies the element. The mass number is the total number of protons and neutrons.
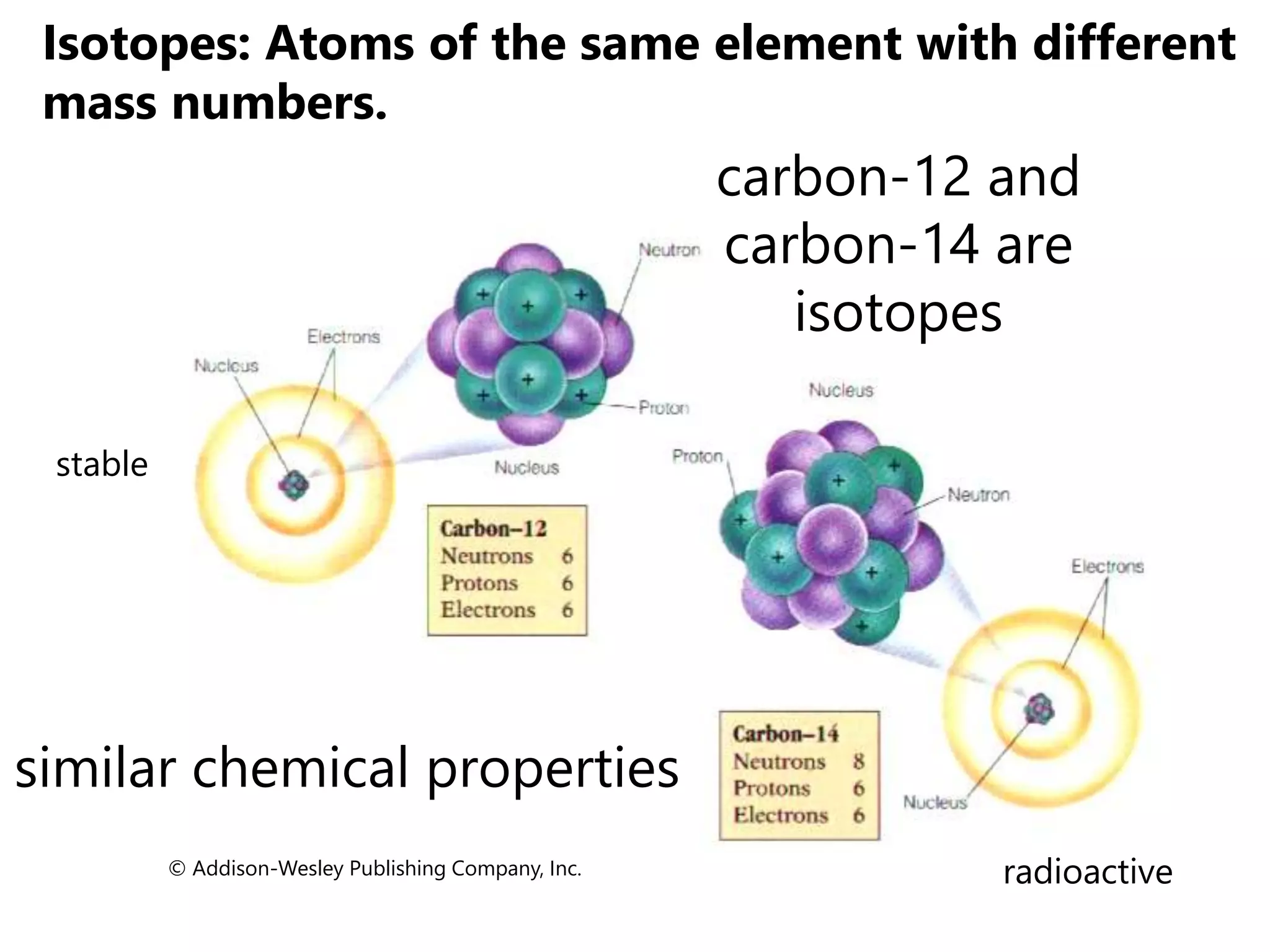
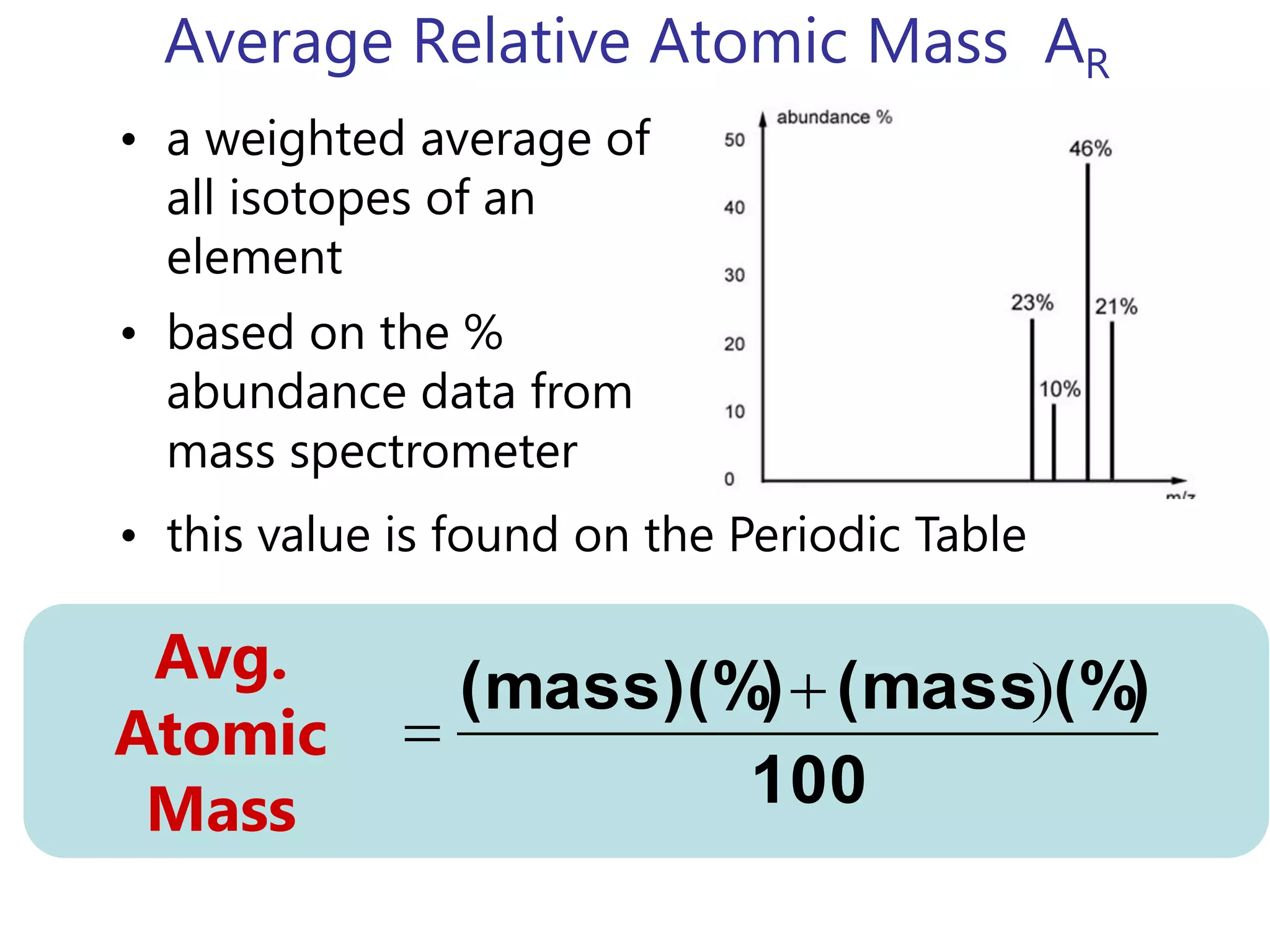
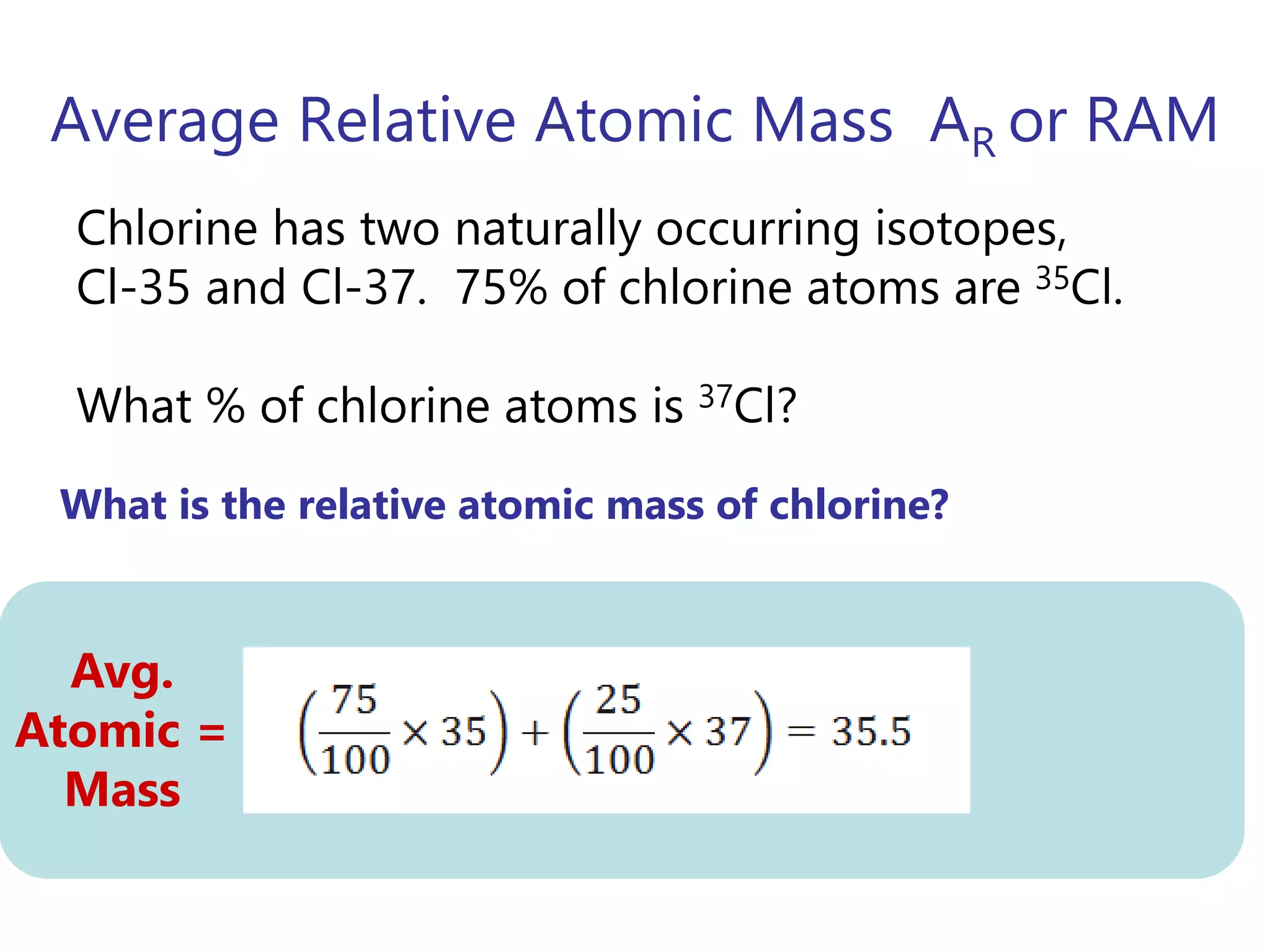
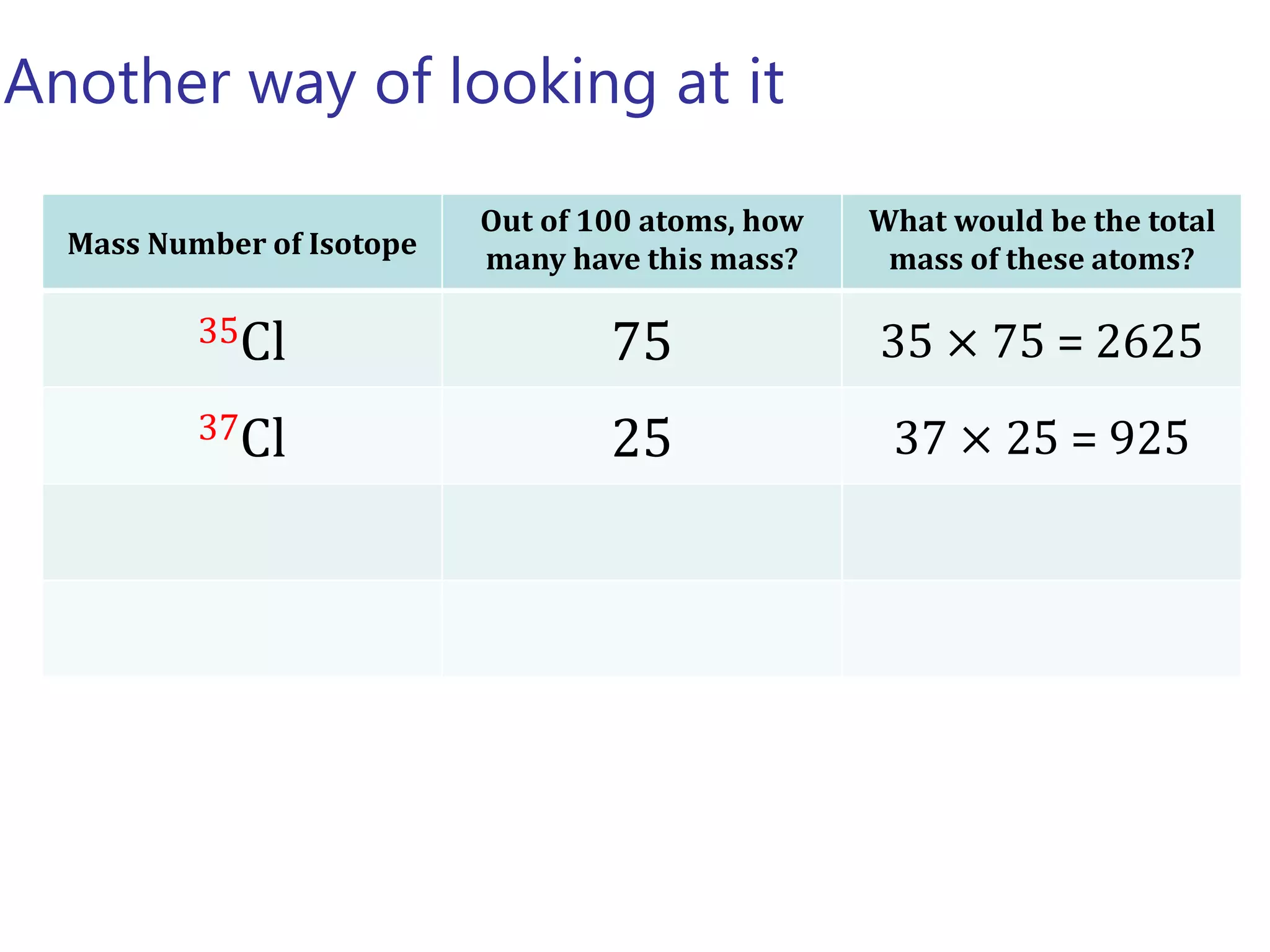
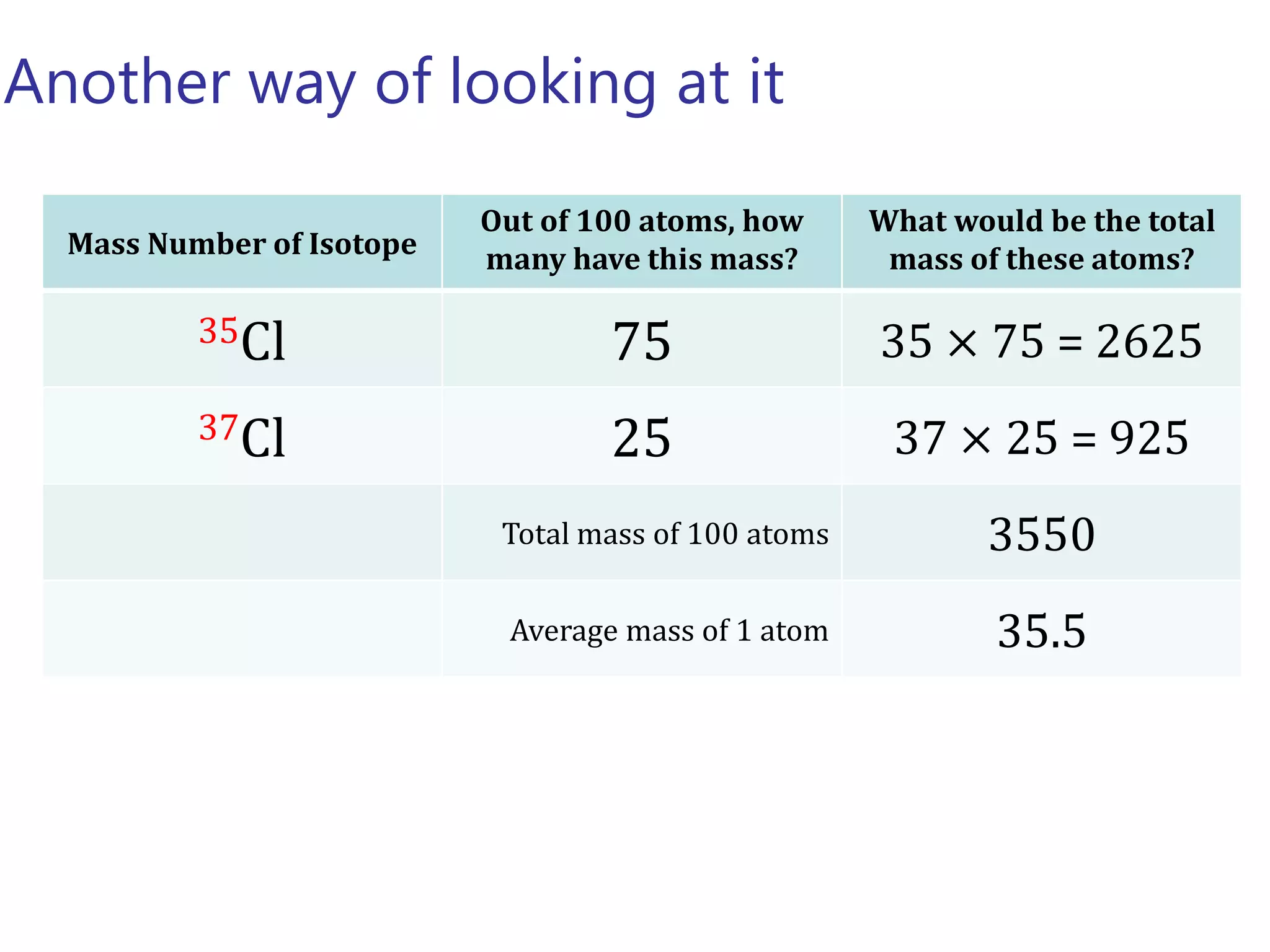
- Isotopes are atoms of the same element with different numbers of neutrons. The relative atomic mass takes into account the natural abundance of isotopes.