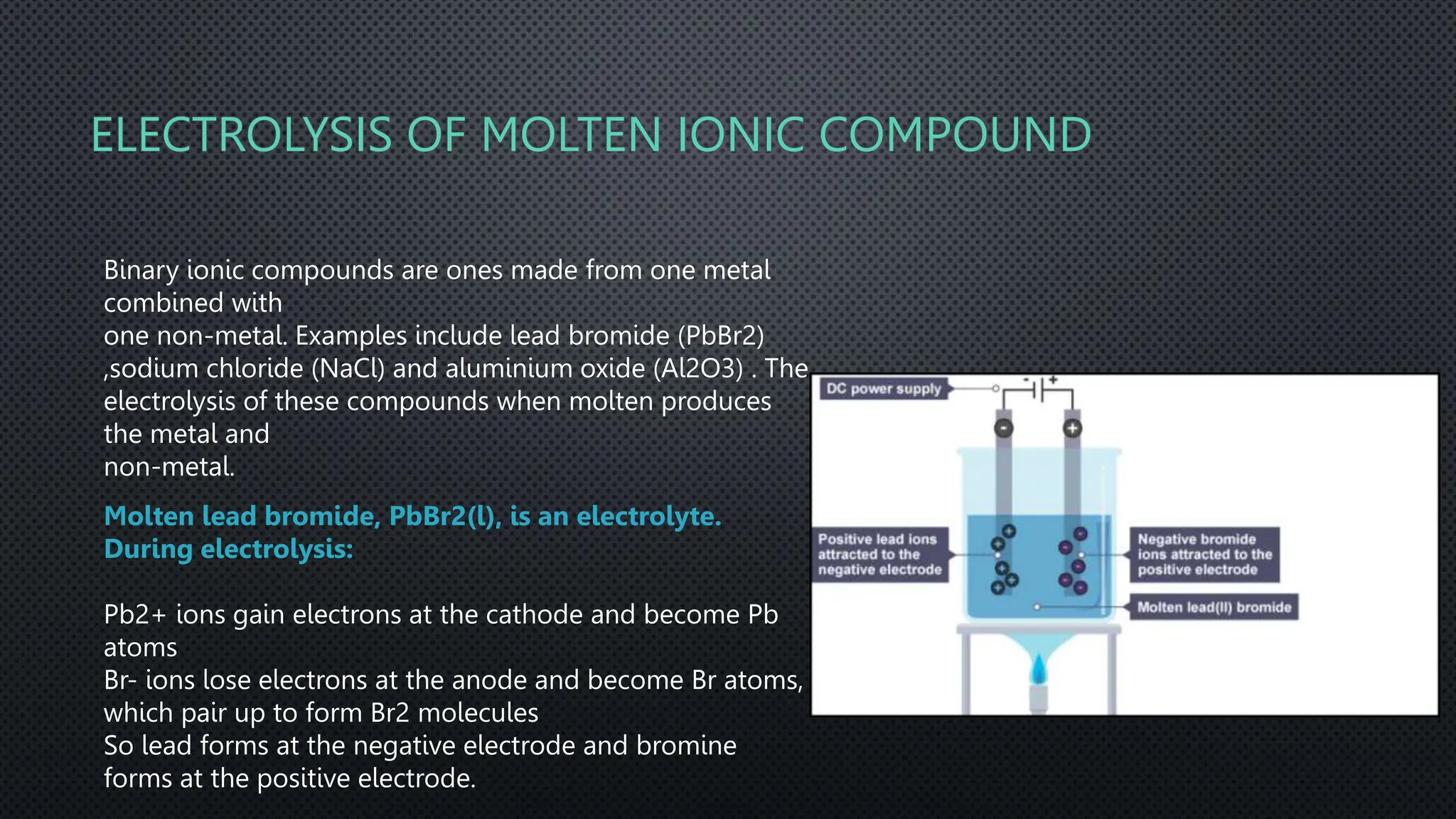
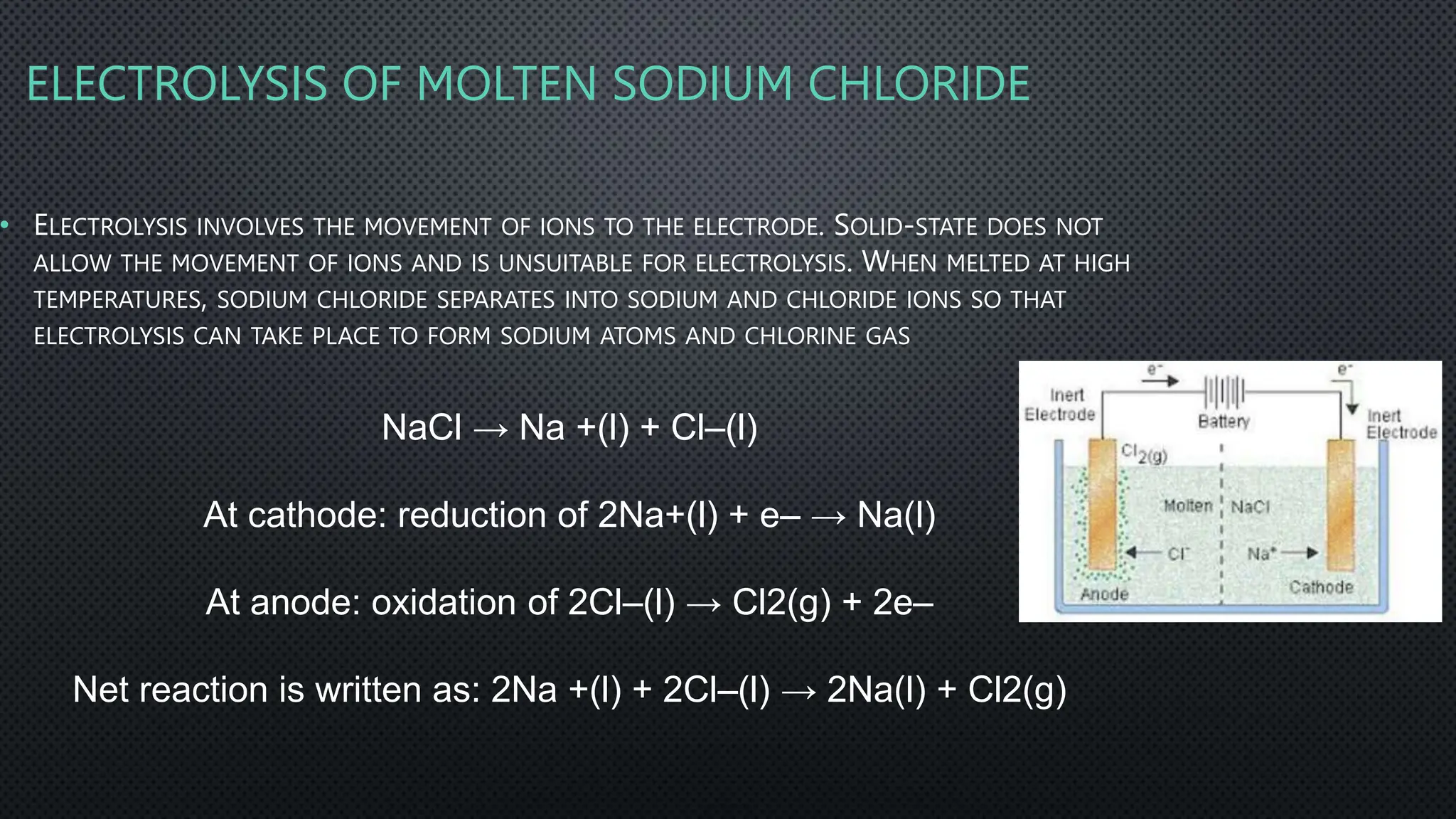
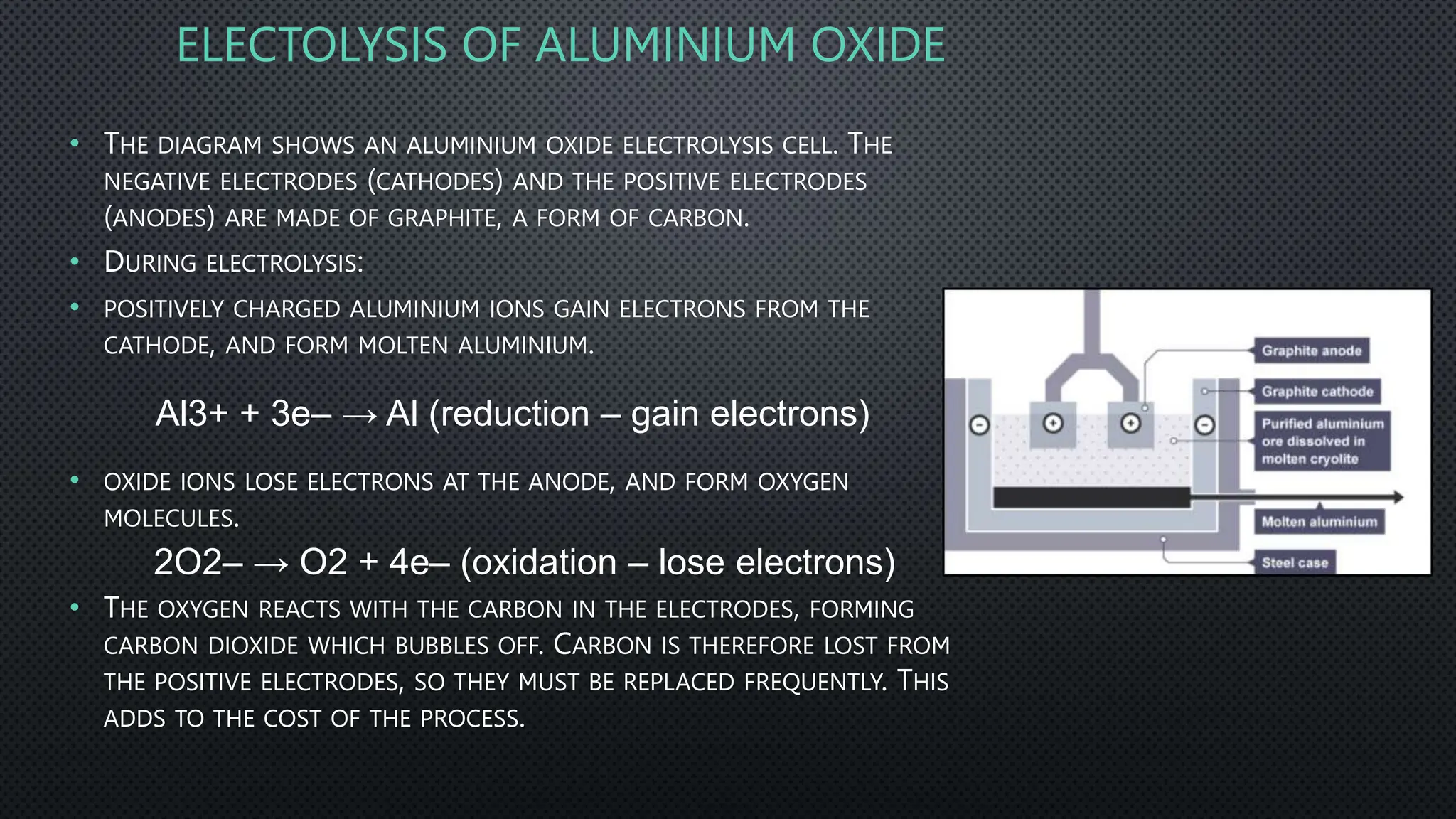
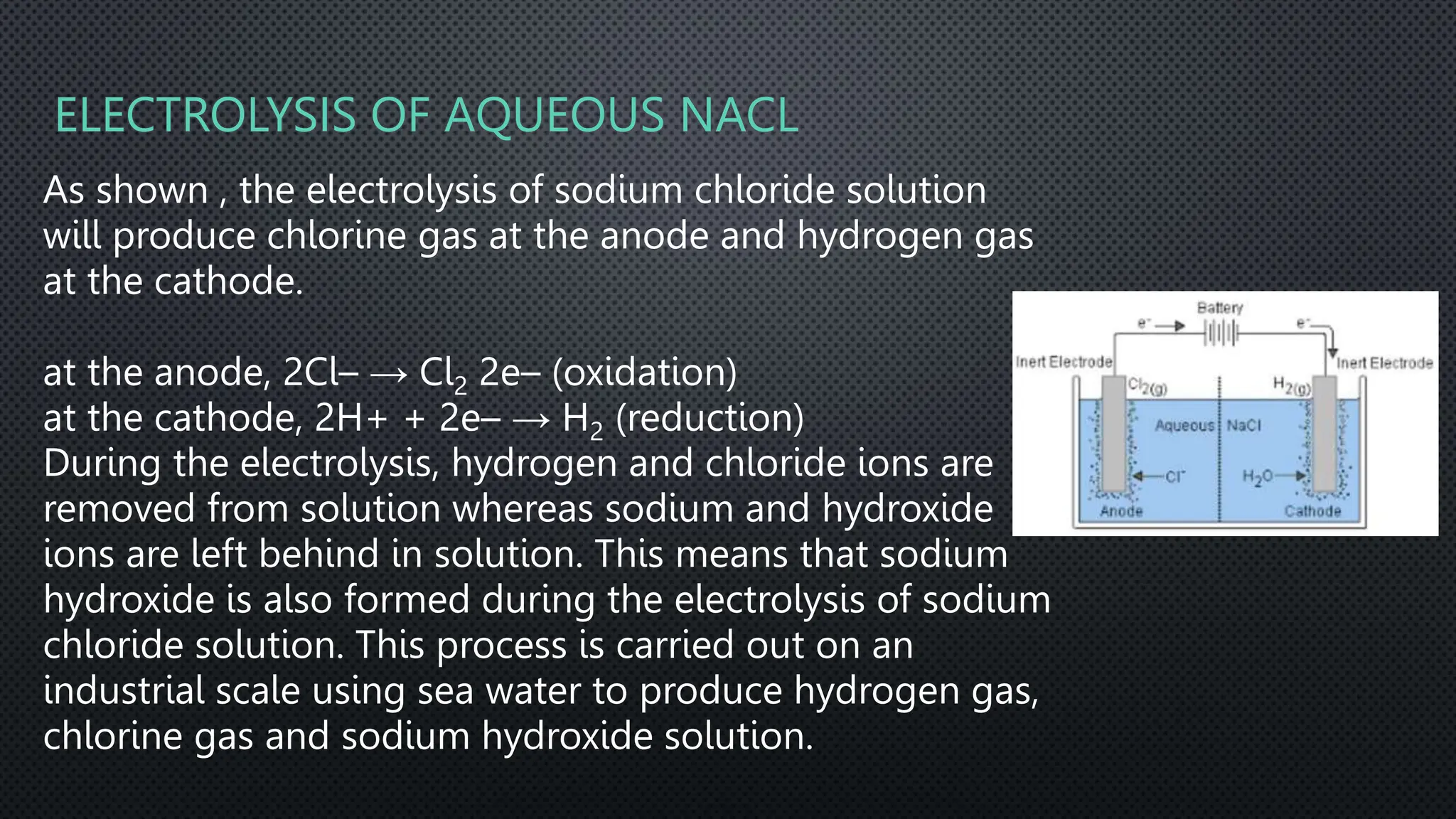
The document summarizes key concepts related to electrochemistry including electrolysis, electrolysis of molten and aqueous ionic compounds, electroplating, and hydrogen fuel cells. Electrolysis is the decomposition of ionic compounds using electricity. During electrolysis, ions are discharged at the electrodes. For molten compounds, the metal and non-metal products form. For aqueous solutions, the products depend on ion and metal reactivity. Electroplating coats objects with metal through electrolysis. Hydrogen fuel cells use hydrogen and oxygen to produce electricity and water.








![Electrolysis is used to electroplate objects (coat them with a thin layer of metal). This is useful for coating a cheaper metal with
a more expensive one, such as copper or silver.
The negative electrode should be the object to be electroplated.
The positive electrode should be the metal that you want to coat the object with.
The electrolyte should be a solution of the coating metal, such as its metal nitrate or sulfate.
Here are two examples.
Electroplating with silver
The object to be plated, such as a metal spoon, is connected to the negative terminal of the power supply. A piece of silver is
connected to the positive terminal. The electrolyte is silver nitrate solution.
Electroplating with copper
The object to be plated, such as a metal pan, is connected to the negative terminal of the power supply. A piece of copper is
connected to the positive terminal. The electrolyte is copper(II) sulfate solution.
Purifying copper by electrolysis [Higher tier only]
Copper is purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper(II)
sulfate. The anode (positive electrode) is made from impure copper and the cathode (negative electrode) is made from pure
copper.
Pure copper forms on the cathode.
ELECTROPLATING](https://image.slidesharecdn.com/chemistrygcsechapter5-231102115057-ea28bf4f/75/chemistry-GCSE-chapter-5-Electrochemistry-pptx-13-2048.jpg)


