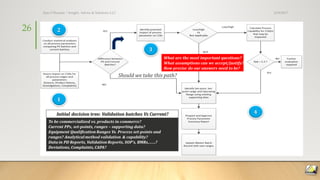
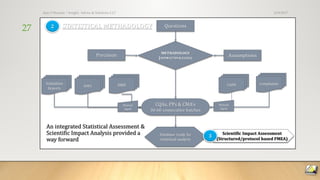
The document discusses FDA trends and new validation strategies in pharmaceutical manufacturing, highlighting the need for a roadmap in process capability. It outlines three approaches for this roadmap: pathfinder, standard, and emergency, while emphasizing the evolution towards real-time controls and continuous manufacturing. The speaker, Dr. Ajaz S. Hussain, shares insights into the intersection of quality assurance, technological advancements, and regulatory dynamics affecting the pharmaceutical industry.



![[Pharmaceutical]
Industrial Revolutions:
1.0, 2.0, 3.0, and 4.0?
• Background: How did the journey
begin in 2000 and progress until 2005?
If you are interested please click on
the video message from the past
• Pharmaceutical cGMPs for the 21st
Century — A Risk-Based Approach
• Today we have an
unprecedented juxtaposition of the
main forces necessary for the US
pharmaceutical manufacturing
renaissance – we are at the Tipping
Point
3/19/2017
Ajaz S Hussain | Insight, Advice & Solutions LLC
4](https://image.slidesharecdn.com/fdatrendsnewvalidationstrategies-170319131931/85/FDA-Trend-New-Validation-Strategies-4-320.jpg)











![What?
• Technology platforms
• Manufacturing with controls [PAT-RTRT partial or full]
• API only, DF only, Integrated API & DF
• Rapid development, manufacturing with controls
• PAT-RTRT with continuous manufacturing
• Other – human behavior motivation & monitoring , Big Data, pattern recognitions, etc.
• New (generic or brand) development Vs. Post Approval
• OSD IR: BCS Class I, Direct Compression,….
• OSD IR: BCS Class IV, Wet Granulation….
• OSD MR: Extended Release
• …….
• People & Organization Development , Partnerships & Collaboration
• Internal mindset shift and collaborations
• Regulator communication and collaboration
• Partnership & collaboration with Suppliers & Technical Experts
• Collaboration with Academia (e.g., NIPTE)
You need to consider
3/19/2017Ajaz S Hussain | Insight, Advice & Solutions LLC
16](https://image.slidesharecdn.com/fdatrendsnewvalidationstrategies-170319131931/85/FDA-Trend-New-Validation-Strategies-16-320.jpg)