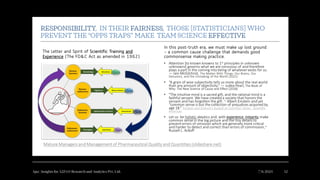
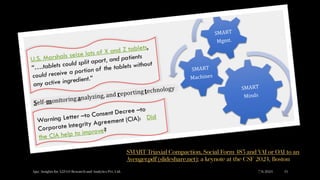
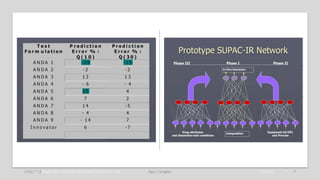
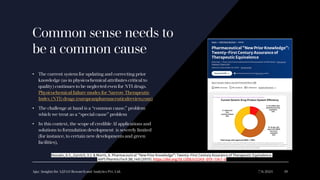
The document discusses the evolution of pharmaceutical product development, emphasizing the integration of artificial intelligence (AI) and the need for improving practices related to data integrity and quality assurance. It highlights the importance of bridging interdisciplinary gaps and applying a common cause strategy to address systemic issues in the industry. Furthermore, it reflects on the challenges of updating legacy knowledge and ensuring the effectiveness of pharmaceutical formulations in the current landscape.















![Mind the integrity gaps in data and wisdom
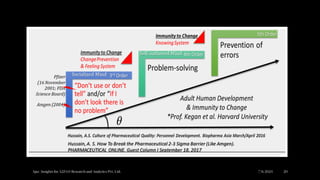
• Decades have passed, and legacy knowledge gaps and errors persist. I am worried that we
are not giving enough importance to these gaps and mistakes and that suspicion of
breaches in data integrity [BAD-I] continues to create significant challenges in both
technical and social contexts.
• Let us consider a risk-assessed approach to “reset” to facilitate updating and correcting
prior knowledge locked in past submissions
• Let us focus on applications of AI to gain a holistic systems perspective on pharmaceutical
life cycles
• A common cause strategy that detects, corrects, and prevents fraud and provides a fair and
responsible way to balance maturity and securing humanity.
7/6/2024
Ajaz | Insights for A2Z4.0 Research and Analytics Pvt. Ltd. 21](https://image.slidesharecdn.com/mindingthedeltaerror-240707093001-988ad607/85/Minding-the-Pharma-Delta-Error-in-the-Post-truth-World-pdf-21-320.jpg)
