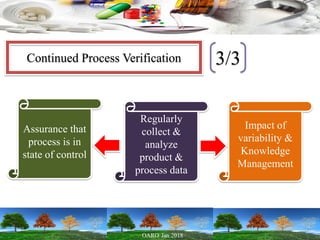
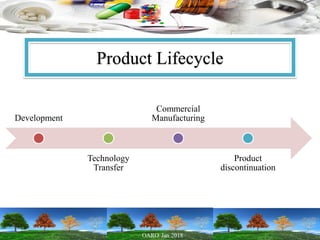
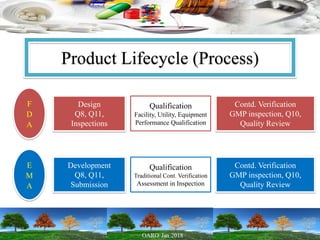
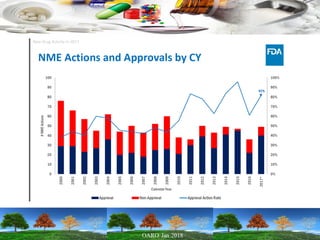
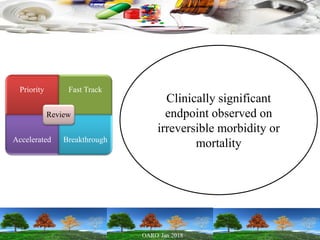
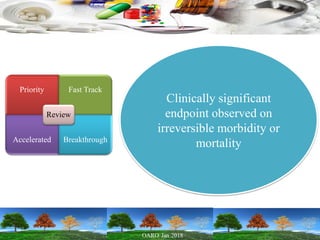
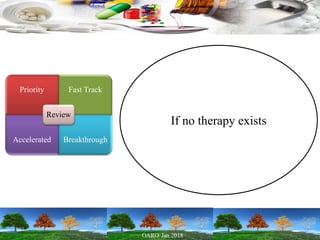
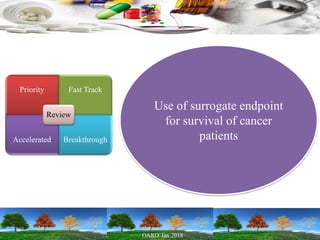
This presentation provides an overview of pharmaceutical manufacturing and regulation. It discusses key performance metrics for the industry in 2017, the importance of safety, efficacy and quality, and outlines the process validation lifecycle. Challenges like inspections, recalls and drug shortages are also addressed. The presentation emphasizes learning, applying science-based approaches, and maintaining a culture of quality.