Here are the answers to the questions:
(a) (i) Sodium hydroxide
(ii) Saponification
(iii) Potassium chloride is added to neutralize any excess hydroxide ions produced during the reaction which could otherwise cause soap to decompose.
(b) (i) X is an acid, Y is an alkali
(ii) Limescale
(iii) Calcium ions present in hard water react with soap to form insoluble calcium salts and water, causing a scum.
(iv) One disadvantage of using agent X is that it could damage fabrics if used in excess.
(c) (i) Ammonia
(ii) N2 +
![gw'5
Pe.vr slo N
| . Ail e:iglrrir:*ent is can:ie.4 out to tletennine tlrt'heat of<li-splar-ernent {a+: lhe re;actk}ri bern'*ea o*,g4rtl.{n[]
surhate stllurion and elcess rir-- gxllvder- 5O.0 crn-t e18.5 aml dr*-i ccgrper{nt} sulphate *'r,ltrticri is 6xlurd int<l
a pi:astic exp. The initi*l teutg*r"*t*re rrf tk stliution is reccrcled *Iier 5 ntinu:*. 5 g rlf er,;t--css aiac 6rc*der is
;ulded int(} a pl;rstic cujr.. The nrixture is stin'eri and tlre lrigh,:sl telmtrkrature is r.*ccrded-
t'he fulloving d:r*a is oirtai**<l:
nrritial teri*gerature tf cc,pper{n[] silph*te soluti<xr : 28.0'tl
F{ighesr temgerattre clthe prirtrue reachesi = 3*.0 'C
{Rel*t.ir,'e iltclrnic *eirss : Cu"64: S.-l?; G- {6: Zn. 65:
{a} E*-:<xl rls t}.'re exixrirrtent- rvlut is traearrl h,r
Sgxeilic h*lt cagr*cit5'c!'&l€{': .11.1 3g ' 'C ill
ria* h*r *i<lispi*reitrl*nt?
(d]
[E lx*rl]
(hl
(ci
r'rige *i ir,ruic equatior: ior the reactiotr.
{l tnarkii
Siate trr* *i-rsr!'ati(llls ir ttre es;xr.'inrent"
C*icr:l;rlc'.
{ii tiie h*at r"elease<i
[€ *t;lr"i<!
(ii! th* uur*txr *lmrli*s {:!t'Lopi}€r qln! su$hlre F:;}ilIcri
[] ntarkl
./
r-rs :ui;fi i: i. i,r,r.irt
hl.:iur F$rr;i1;- 5141;g
i{otej t 4:i;1,'u. iilj ai; :.ia*g5 gi](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/75/F5-exercise-1-1-2048.jpg)
![!
i
s, ,F
l.
' L A i.
v'
i
(iiiI the !r*nt *Ftlisplacernent
{d } 'li.r, exces:s einc gxlrvder. is usetl ilr this experiment?
,t
ih..
[2 uewL:]
{R ra*rk}
{B Dr:nr, Ibc ener:+' level di+{ir:*n lbr tle te*etir-rn-
[) irl;ari"]
2 Sq
"trrt
1.0 rmrl dnt-'tsrdiurn ii"rdr*sidr srltuticn is ;rcurrd iutt'r:l trx'li-r'st-r r.ene cup. Ttre Sxll-ls; rerc c*p is
tfren pl;ued irrtc rr i:eahrr: *^s sl:cer: ir':r Di*g,rurn 5
Itc!.r st.r lerie ctti':
S*diirr:t !rg,":irt'r'xide s*lulirl* +
h,r<irociiicric acld
lli*gruun -i
14,<lnx-hl*ric :tcid is adde<! tt-r the i$ cirt
r
sr.diirnt ir; dr"oxide :rrtid tl:e ten'l!:Eftltla"e inc:eme i.s r**rlrrled. Thea
ih* h**t enel-s: rele:ased ir.calellarrd. T'h* exp*r'ir*ei::t ir re6reei*t tr"r a,riding di*ir**rt r.elurx*s *,1
kl <irtrcirlcrie ti) tlic :;8 ctn'- *i s*liLrm t+drcride. Flir ru-srrli-s ::r't t*txii;lted *s skrin !-rel<lr: .
Vsf urnne of, rrsdliurnn hydlrtr,rielle
sotrurtisn' i cmj
llellsrme taf h1tl1rocfal*r:ic acicli
:rdc,ied i crn''
f,[elt energ-t relenstd. i [iJ
50 l{! i.x
JO lc! 2.7
50 :{:} 3.4
5{'} {ti.} 4r.5
JO JO i.6
j,fi 60 5.6
5{'} 7{} 5.6
Plot a
-er*pt: lr€at enery+, trltases: :rrErir:si tolitr*e *l!'ildracblqrdc acid atirh:d.
fi nl"tri..i|i
{t'r} {i} en^.F{i *a rlie EErh- detennilre tire rclar*e cfhrtlrcoai{c{ic rnqiiirerl iirr c'trnr,picte
neulr*-lisati<u: *itli -i{} oilt !.0 rrr<rl tlnr"i srrdiurrl hr<lc}si.:c
[3 rn**]
{ii } Fnu,r 'orrr ans*q'iu ih}, {i}. c*lcul*ir tir€ qx?nc€ntndion olh,rdrr}chlosk acid inu:d
ir: nta'ltlin-s-
p.eoor.ri-r]
-._-]
{a}](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-2-320.jpg)
![{a} 1&$' the cr}Htent in the pol-rsryene cup has to be stiri,ed rxurcinuausty thx*rghrwt the
exg*rit*entl
{d}
[l ma&]l
released bec*,rnes c$fxtant a[ter 5& ttra] cFhr'<1n+clrttlric racid isExplain brie{I.1, rvh; the heat
addecl.
{.c} ql!
[! m*:kll
Chlculate the he*at" errer!i-r,' rel*aeci *Jreri I H'n<lle s'll'slditrln l4tirtlxide is r:eutrelizeEl
tl-r' l1r'tl xlchlelric ;*-id "
{ii}
[! mark]
Drarv the €ner:* level di;rgru:n lilr the reacti{}$ thxt {lrxHr-r'dl in tlr* p<ll;s4,rene cup'
p r:aawll^tll
ilt: anrl{lrer eiiperim-rrl S{'! cr*3 !.{l nrc'i tft:r-r srdicr:t hldr<r,ri<!e s<'viutkln is arride<l *it}: i0 cr,cnt i.{.}
rurcl drut-r etliantlie acid.
{il P'ri:dict ahe iie.-tt €rre{9.} :eiearseri
$ rruwkll
Cire a leavrrr lhr rcrur':urster iri qti t,il
[2 umrL-s]
{l }
i ttl](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-3-320.jpg)
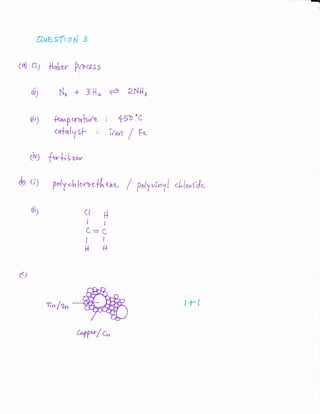
![.3" Diarlgam 3.I strons *re rn*nrfacture<l <vl*mr**nia.and sulphalic aci<i.
Amn*q,firiur,& villphrate
Diagryr*.t.!
E'aset! rya Di*Ealn J. i- ansrver the tollorvillg questl*ns
{i} State the n*rrae olll-rlcess l.
Nt *mrt il
(ii ! l,,rite tlre cherni+ri equat.icn Rlr the re;r*ti<.*r Kl' pmd*cx arntmria in Frr.rces n
p m;arfts]
{iii} nn P'racess U. sulphw: di*xid* is rsrctet'! $ith ex},gen tc p*rduce *rlg:b*r <iiorkle.
Stl:ite tlr€ t€rrtperuture and:he L';4.1$:-st rreti iri tiris r'etxetirlli-i.
[i *rrrurL:]
(i, ) St;lt+ <}nr rrs^ tll. :illlrnoniuL',r sulplrlltc.
[] rrrarllj
{!ri Dia$ir*lir, 3.1 shr:iBs * slr:uclural {qlnn*ii} $la tx}i.}}ii€r..
clHqr&iclFretg{,
ilt$t! [r I
-c-c-c-c-c*e{*e *
ffrttE rt t
HF[HE{H}.TF{iT
*iagr:ar* 3.2 I
{i} State the n*r:re oltiie 1*l1nter in Di*gru* ,3.2
[! nurk}
{iir Dr*t tlre stnrctural lirnnula lcrrhe r*orxnr,er in Diag.rarn i-l
[{ rua*]l
{{} Er*l:ae is a* :all*rr' *,fcoplxr, Dr:lu, e }ateletl clia;:rane te sirc,*' *€;ir1-;}ng**!€r::: e,l*:.**ts li:i tx-c;r:ae.
[2 xwulsl]](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-4-320.jpg)
![4" T-h* Ib,ll*rving eqrmti{in $rtlrvs trcrr soep {*n he pregxa**'i"
Jrakle *il + ix!.tassiurri hlttr<lsinte
-.subctance f + vlE-l
{*} lii} S*rLte lhe narne olsubstxnce /.
[E r*arLl]
{ii} Skele tlre n*ne olthe 1}rc{es in the ;rtx:re reaclion.
tl selrt<.li
{ii!}Explain *tr-r'lxltias-siurn chklr:ide is adtled duringr tlie pr€}'!:iratiori $lst}*p.
{! aae*}
iblT*tl elearrilrg ag-e{La X antl Y, ;ire usd t* rvas}r clcrhes i:i siver *'alrr arxl sea tr,altr- fire p:sclts *biaineul
*ie sholvn in T*ble 4 telclrv.
Clte*,ni*r,s a,selg'f, Rircs rv'*ter Se* x*ses
x llqr'es txr,l linrrt s-'l** lhts nsr,! fi':ssl scr:ut
Y Ihes rx:lt. {brl* scuru Fonres sct*rr
T;rble 4
iil' State tlie tr:,pe $.!'cleiurlillg agents X at:d Y.
p uaari.-sll
{iii State tk.: r:;air1€ clll'rc s*r* lim*td ii'Y is s*riitail geara!€.
[t rru*.id1
{iii}St:ete th€ trr'iry i<vs presart iu s* u*!er- !h:rt r::luse tbc iitrtatitlu s,lscritl in V
p n:*rlsll
qir,!d!ve or:e .lisxrlra*mge of ttsirlg the cleaning a::.erit X
[l r*rt]](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-5-320.jpg)

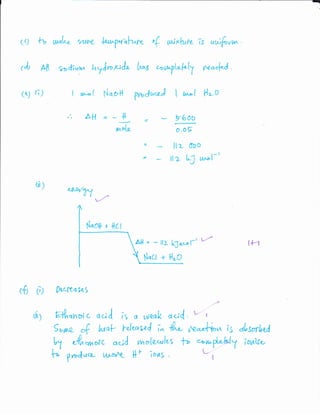
![(e)
JI
G IM4VL
fvar,t-al
91rt Q- 4[l L.o1fr/ Ut) Se{pl,,..f< l"^a e*u,^yUazll
<[, ryI -/
z4 + Q1SQ+
6,ha>1toN L
Aft = - 94 {.J*" f,_/.
450+ t C"
EJ
t t.r
6Qyt,
5oCJ
tlr )
w"l
EYal'ou,
t'.1.o H :
I r,rnol
o,o5 tol
ll ct
I r,tr.-{
D' 0 5 r,r^tf
Ut-e- r.t^-r>(t
tdDo
b n g,/"upt ?^f*
cran
]
N qatt
woL N"D$
+ l{cr
= y-y
liiruo
Nqc I
= (,Dg)
l6D o
t+ I
{- llrO
= 0,a9
r^ rt. X lop o
V
o.og x {Ooo
M,,l+c{ '](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-7-320.jpg)
![-
&uesT( oN +
(41 61 Gy<-r,nl
di 9alo,orfr.ofiura
dii) {r [eug;]-4v 1fo
&(i) x'bkrXs^F
L,'l ' toay
soay / h fledu*, srl"biliJ-, 4toy
(il
) t'qy.as,,rM #eqn*e / cqlciut, s{.q&{&
(iii ilMlyreliulr^ ion q^d cq l.;uu, ;o^
f M?* o^J Co'r
c,Y; ro tn - Lru J
"7
rad obl-s-](https://image.slidesharecdn.com/f5exercise1-130918221016-phpapp02/85/F5-exercise-1-10-320.jpg)