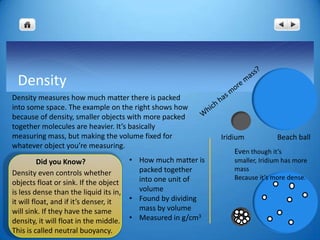
Archimedes was asked by the king to determine if a goldsmith had cheated by adding silver to a gold crown. To solve this, Archimedes needed to calculate the density of the crown by measuring its mass and volume. However, he could not directly measure the volume of the irregularly shaped crown. Through his principle of water displacement, Archimedes was able to determine that the volume of an object is equal to the volume of water it displaces. He submerged the crown in water and measured the displaced water to find the crown's volume. From this, he could calculate the crown's density and compare it to pure gold to see if silver had been added, solving the king's problem.