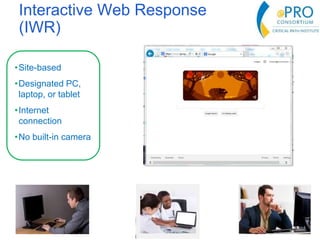
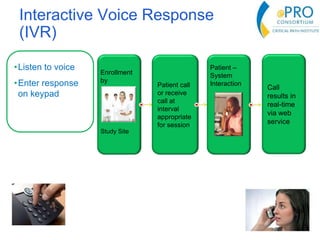
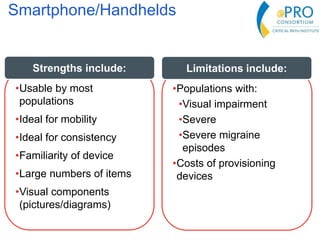
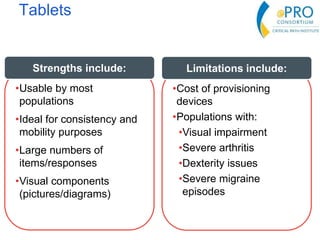
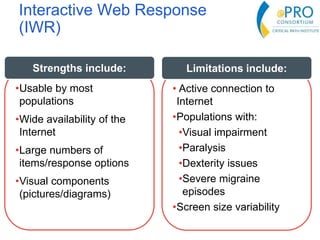
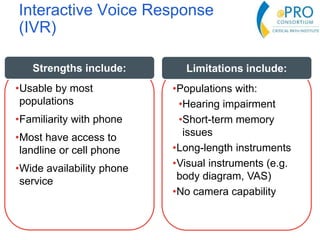
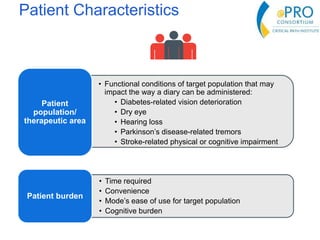
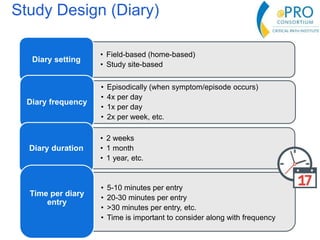
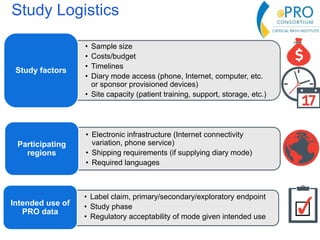
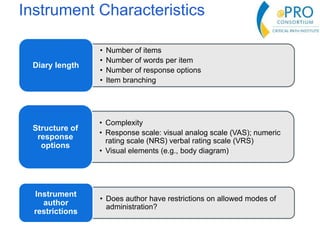
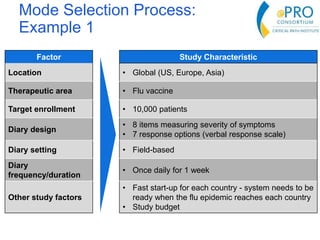
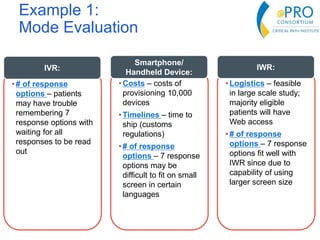
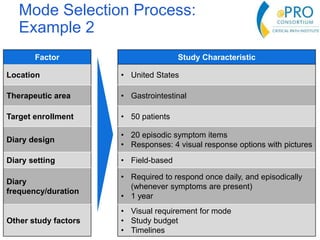
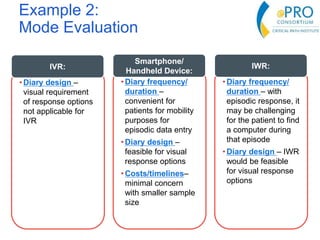
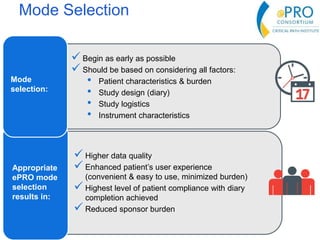
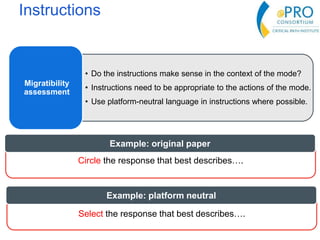
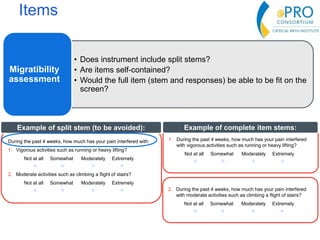
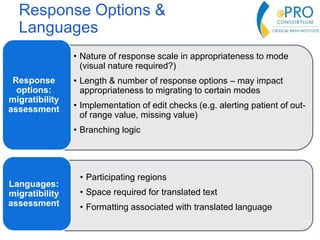
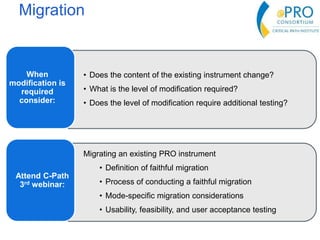
This document summarizes the key points from an introductory webinar on electronic patient-reported outcomes (ePRO). It discusses the current modes for ePRO data collection including smartphones, tablets, interactive web, and voice. The strengths and limitations of each mode are provided. A process for selecting the most appropriate ePRO mode for a study is described, considering factors like study design, patient characteristics, and instrument type. Examples of applying this process are given. Lastly, migrating existing paper PRO instruments to electronic formats is introduced.