- Epoxides are cyclic ethers with a three-membered ring that are commonly prepared by epoxidation of alkenes or from halohydrins. They undergo ring-opening reactions under acidic or basic conditions.
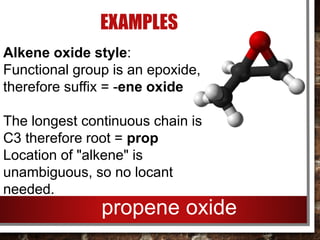
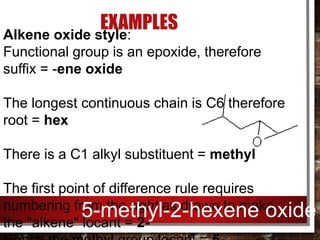
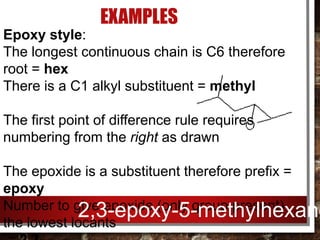
- Epoxides can be named using either the alkene oxide or epoxy style and their reactivity is due to the ring strain of the small three-membered ring.
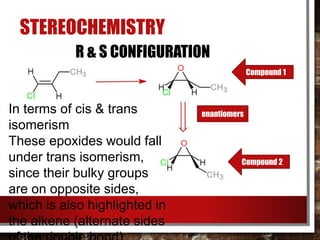
- Stereochemistry of epoxides involves assigning R/S configurations and determining cis-trans isomers.