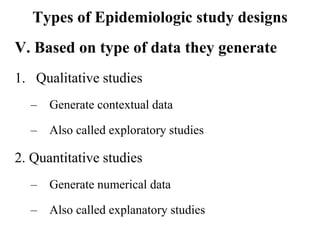
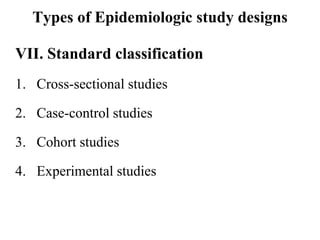
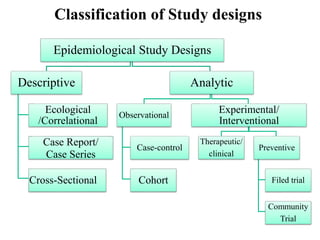
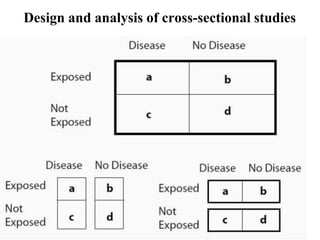
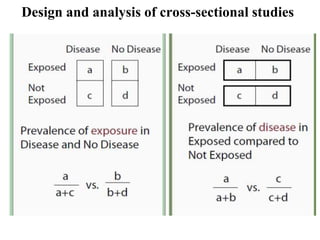
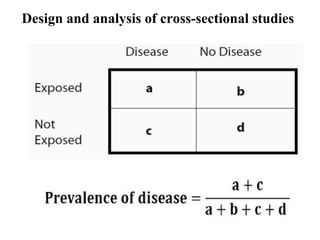
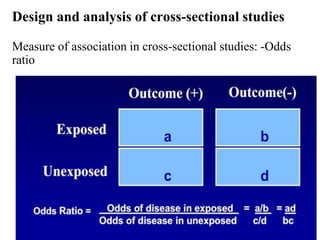
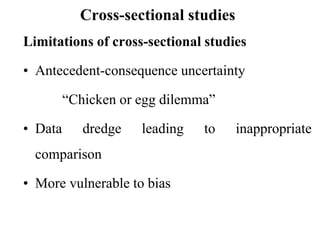
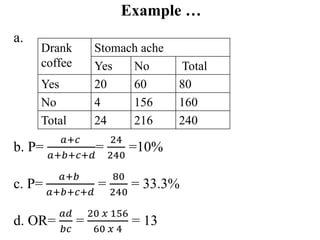
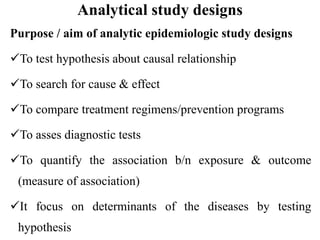
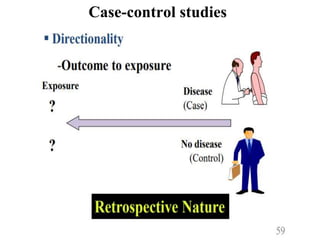
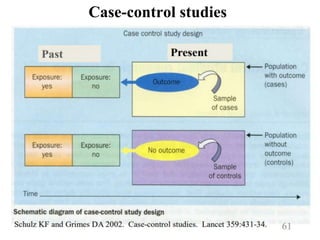
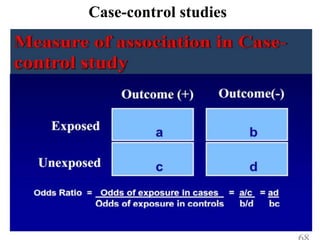
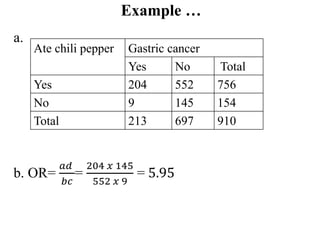
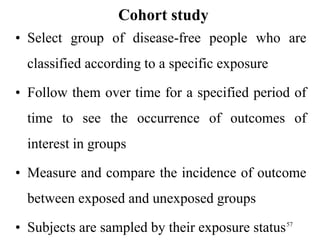
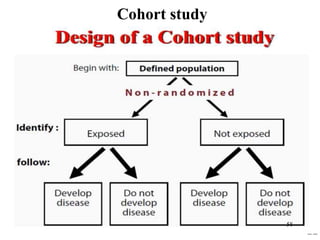
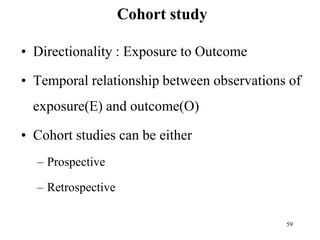
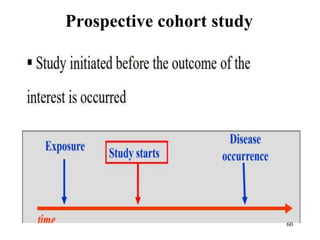
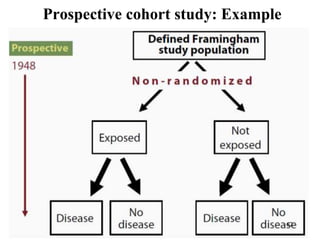
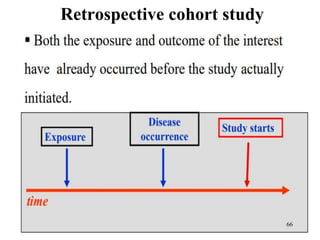
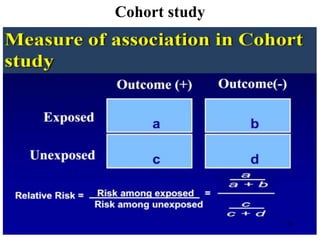
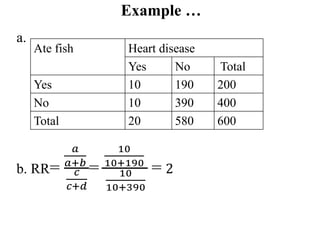
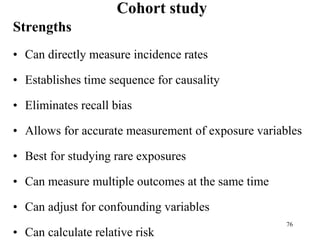
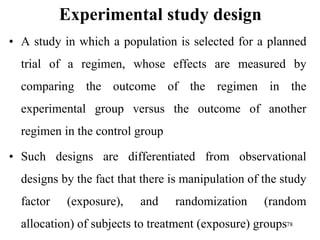
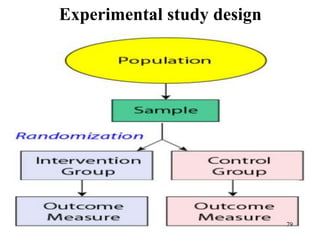
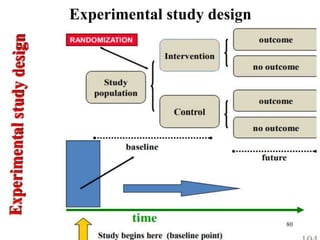
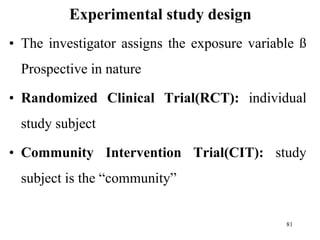
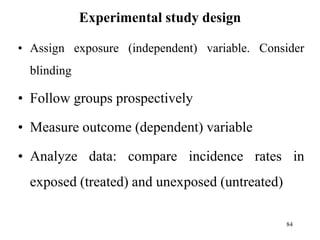
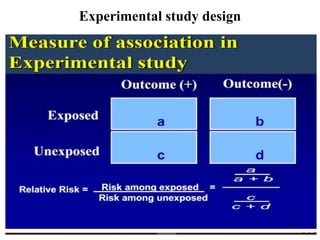
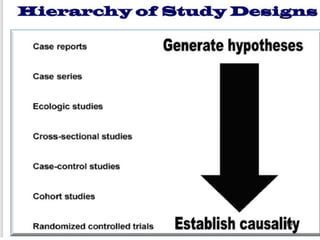
This document provides an overview of epidemiological study designs, outlining their definitions, types, and classifications. It distinguishes between descriptive and analytic studies, observational and experimental studies, and further categorizes them based on timing and data type. Moreover, it discusses the advantages and limitations of various study designs, such as case reports, cohort studies, and case-control studies, providing examples and considerations for each.