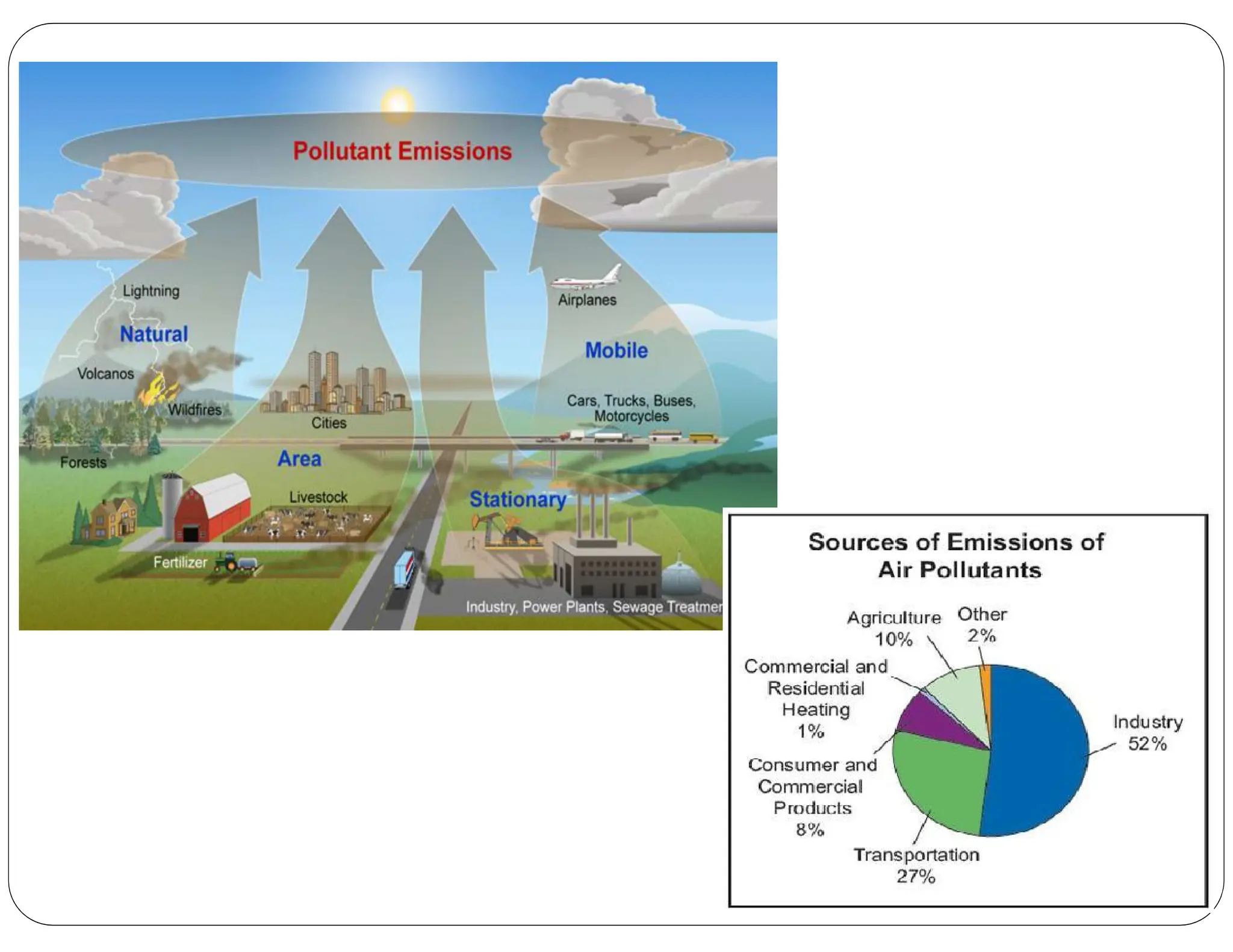
Particulate matter (PM) consists of various airborne solid and liquid particles, with PM10 being inhalable and PM2.5 being finer particles with distinct emission sources and compositions. Major contributors to PM pollution include combustion of fuels, industrial emissions, and natural sources like wildfires and dust. The document also discusses the adverse health effects of particulate matter and the importance of ambient air quality standards to mitigate pollution.