Embed presentation
Download as PDF, PPTX
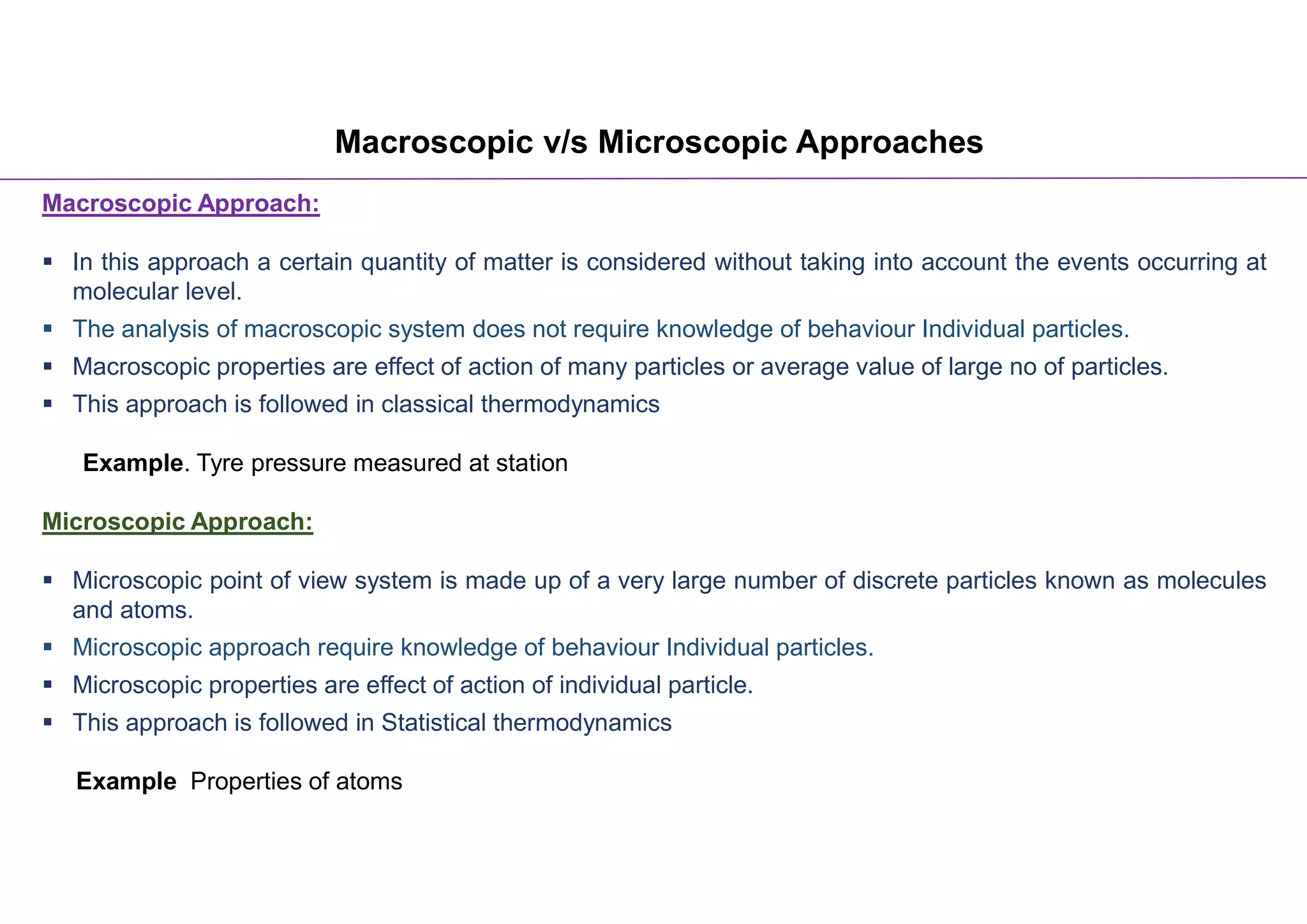
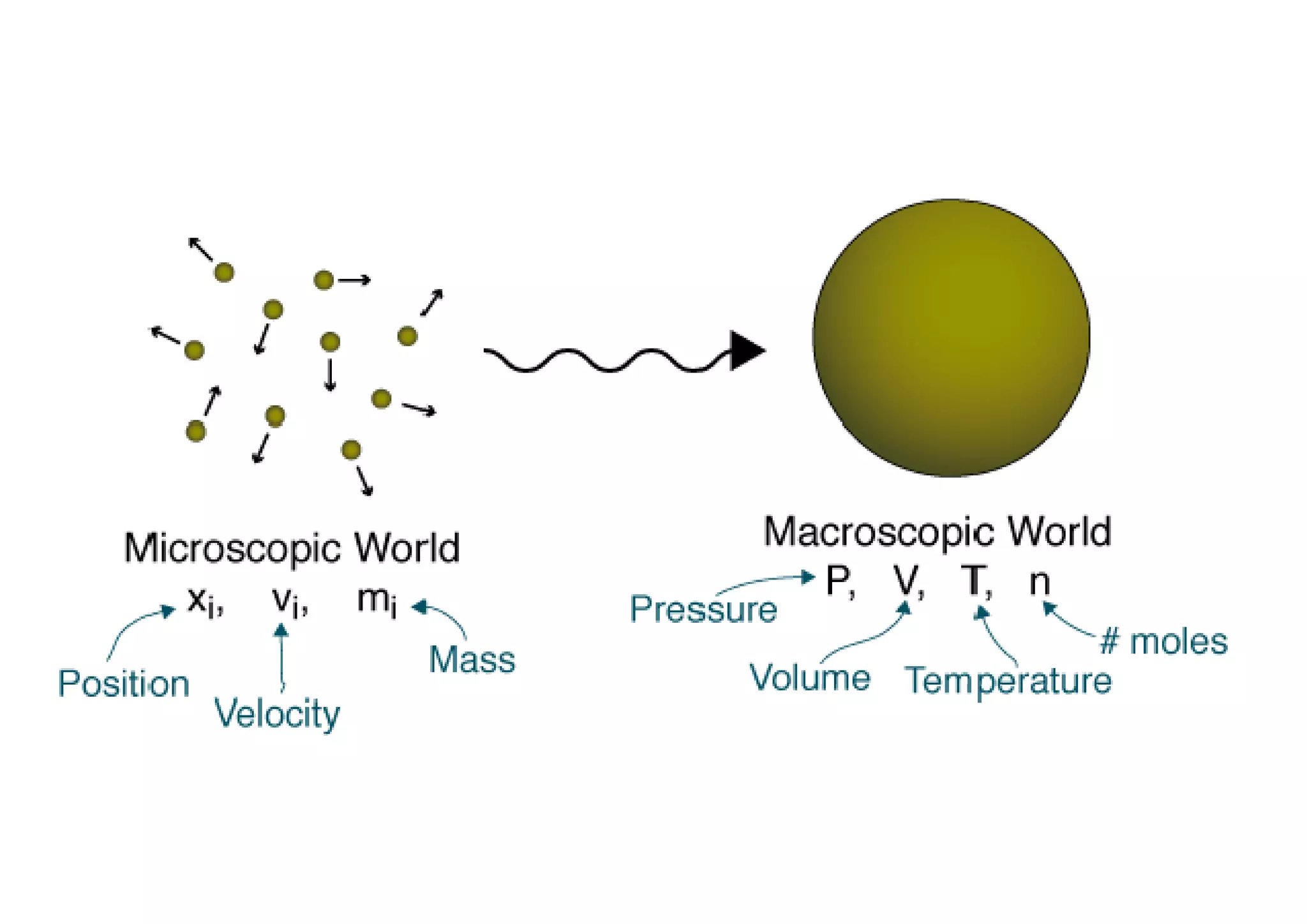
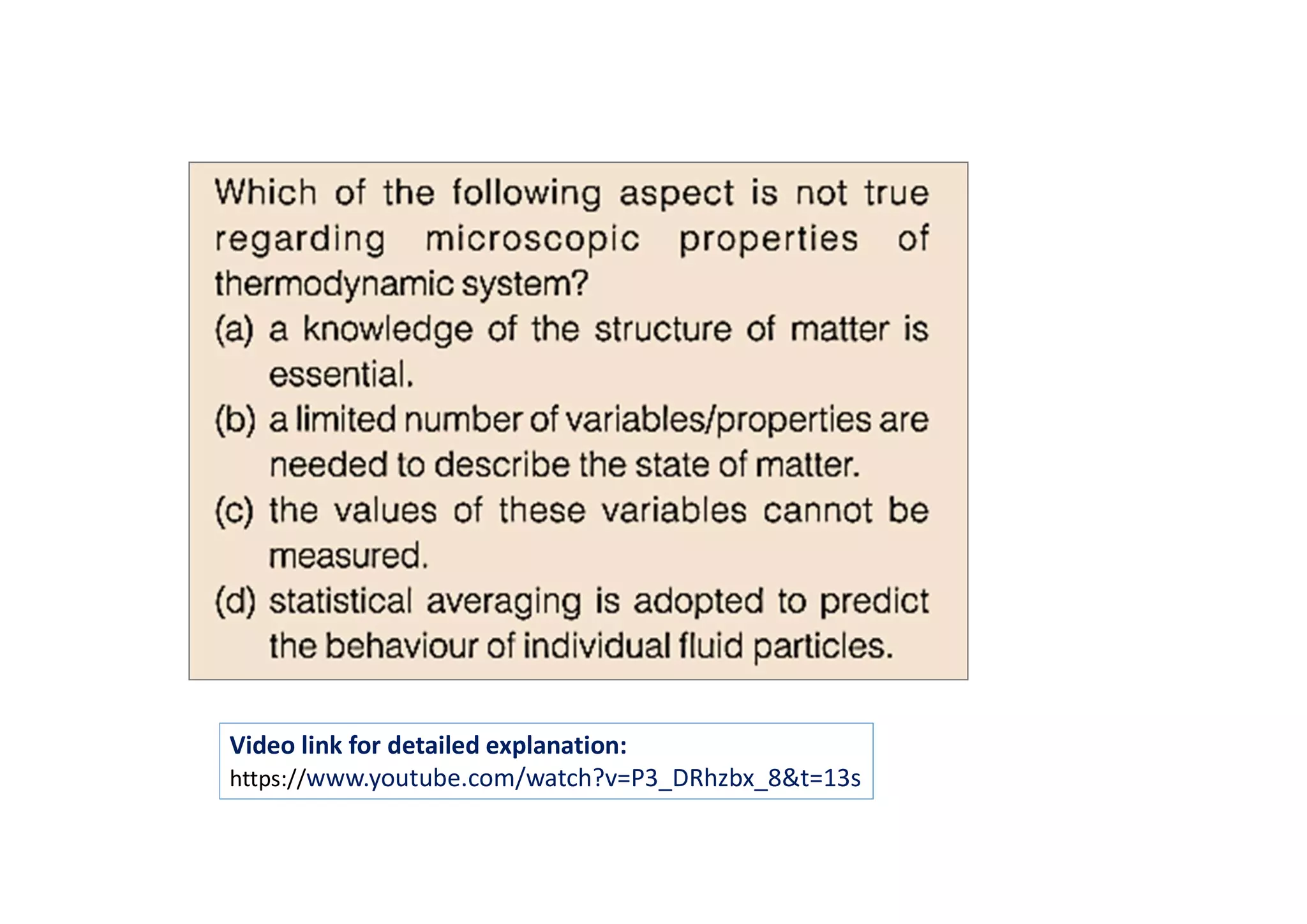
The document discusses macroscopic and microscopic approaches in engineering thermodynamics. The macroscopic approach considers bulk properties without analyzing individual particles, while the microscopic approach examines systems at the molecular level and requires understanding particle behavior. Examples are given of tire pressure measured macroscopically and atomic properties analyzed microscopically. A video link is also provided for further explanation of the key concepts.



