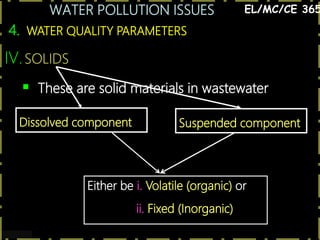
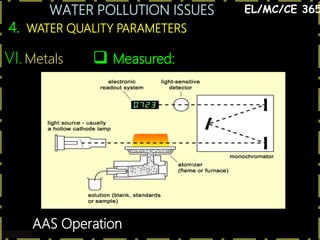
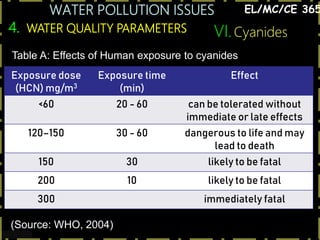
This document discusses various issues related to water pollution. It begins by describing different industrial uses of water, such as for cooling, processing, and transport. It then discusses how industrial activities and mining can pollute surface and groundwater through runoff, spills, and aerial pollution. Next, it examines how pollution can disrupt aquatic ecosystems by overloading their self-cleaning capacity. It proceeds to define various water pollutants and parameters used to measure water quality, such as dissolved oxygen, organic content, nutrients, and heavy metals. Finally, it analyzes specific pollution problems including deoxygenation, acid mine drainage, eutrophication, and heavy metal contamination from sources like road runoff.