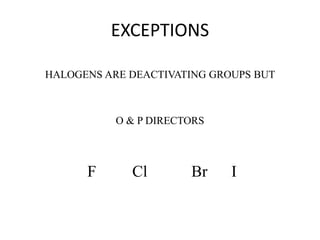Embed presentation
Download to read offline

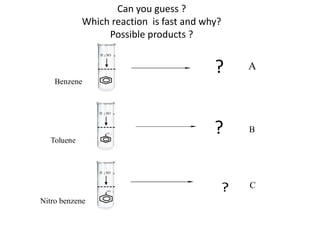


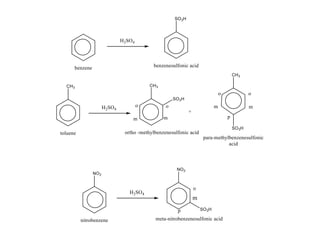


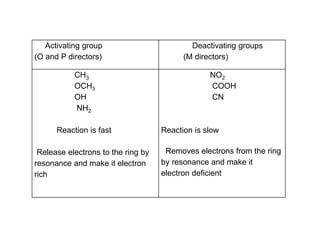
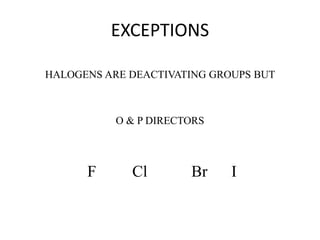
This document discusses the effect of substituent groups on the reactivity and substitution pattern of benzene. It notes that: - Reaction A with benzene is complete within 20-30 minutes, producing a single product. Reaction B with toluene is complete within 1 minute. Reaction C with nitrobenzene takes 2 hours, indicating substituents affect reaction rate. - Activating groups like alkyl, alkoxy and amino groups make the ring more electron-rich and reactive by resonance, directing substitution to the ortho and para positions. Deactivating groups like nitro, carboxyl and cyano make the ring electron-deficient and slower to react, directing to the meta position.