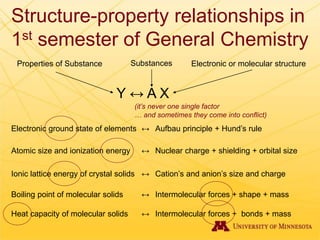
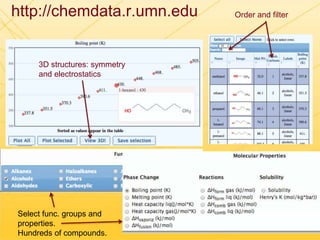
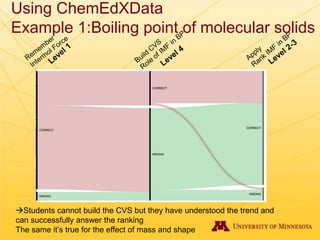
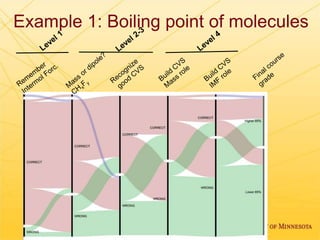
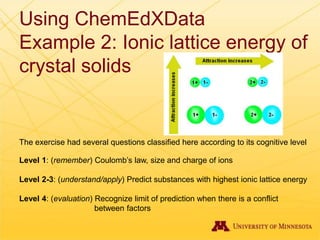
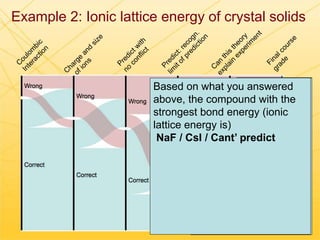
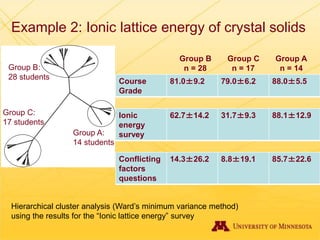
The document discusses using Chemed X data to enhance higher-order thinking skills in general chemistry, emphasizing the importance of teaching students to evaluate conflicting influences and understand complex relationships in chemical properties. It details specific examples and cognitive levels related to boiling points and ionic lattice energy, illustrating how data-driven exercises can facilitate self-regulated learning. The findings suggest that students who master concepts like control variable strategies achieve higher course grades.