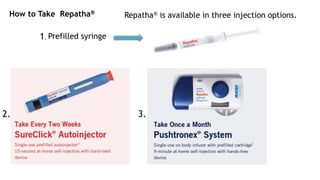
Alirocumab (Praluent) and evolocumab (Repatha) are injectable medications approved for treating heterozygous familial hypercholesterolemia and atherosclerotic cardiovascular disease, serving as adjuncts to diet and statin therapy for lowering LDL cholesterol. They work by inhibiting the PCSK9 protein to increase LDL receptor availability, thereby lowering LDL levels in the bloodstream. Both medications can cause serious side effects, including allergic reactions, and have specific dosing regimens and storage instructions.