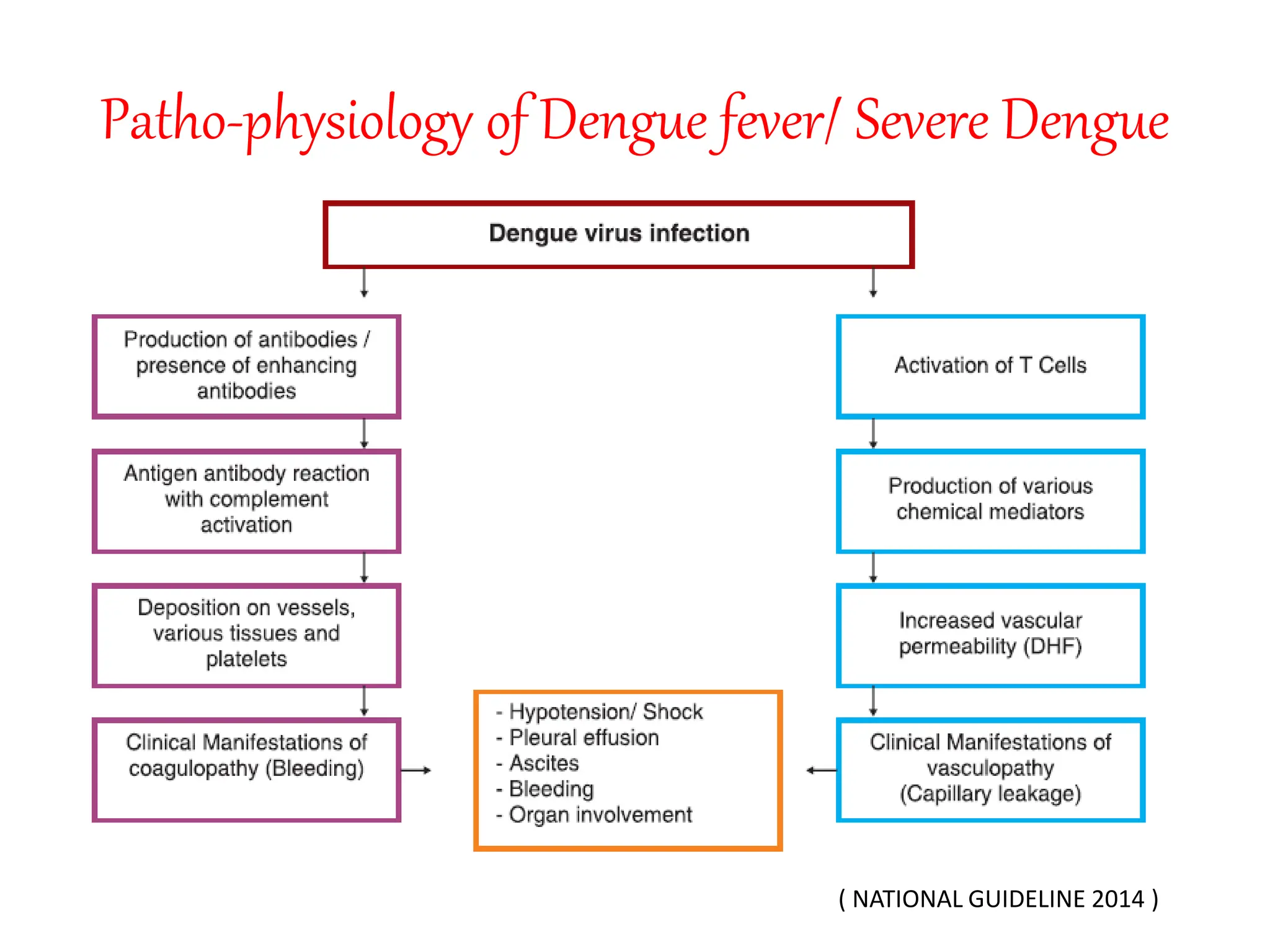
Dengue is caused by four serotypes of dengue virus transmitted by Aedes mosquitoes. The document discusses the pathophysiology, classification, clinical presentation, investigations and management of dengue. It describes the three phases of illness - febrile, critical and recovery phase. Treatment involves symptomatic relief and careful fluid management to prevent complications of plasma leakage and shock. Hospital admission is required if warning signs or severe symptoms are present.