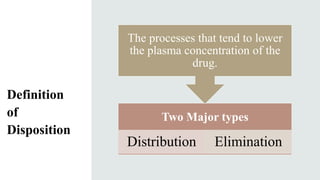
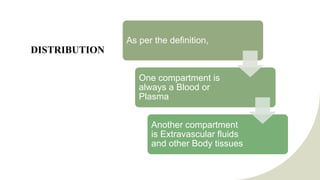
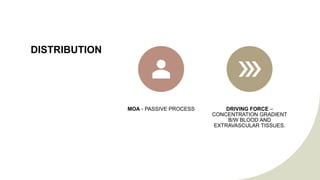
The document discusses drug distribution, explaining the processes of distribution and elimination that affect plasma drug concentration after systemic entry. It outlines factors influencing drug distribution, including tissue permeability, molecular size, pKa, and various physiological barriers. Additionally, it emphasizes how drug ionization and physiological conditions impact drug entry into cells.