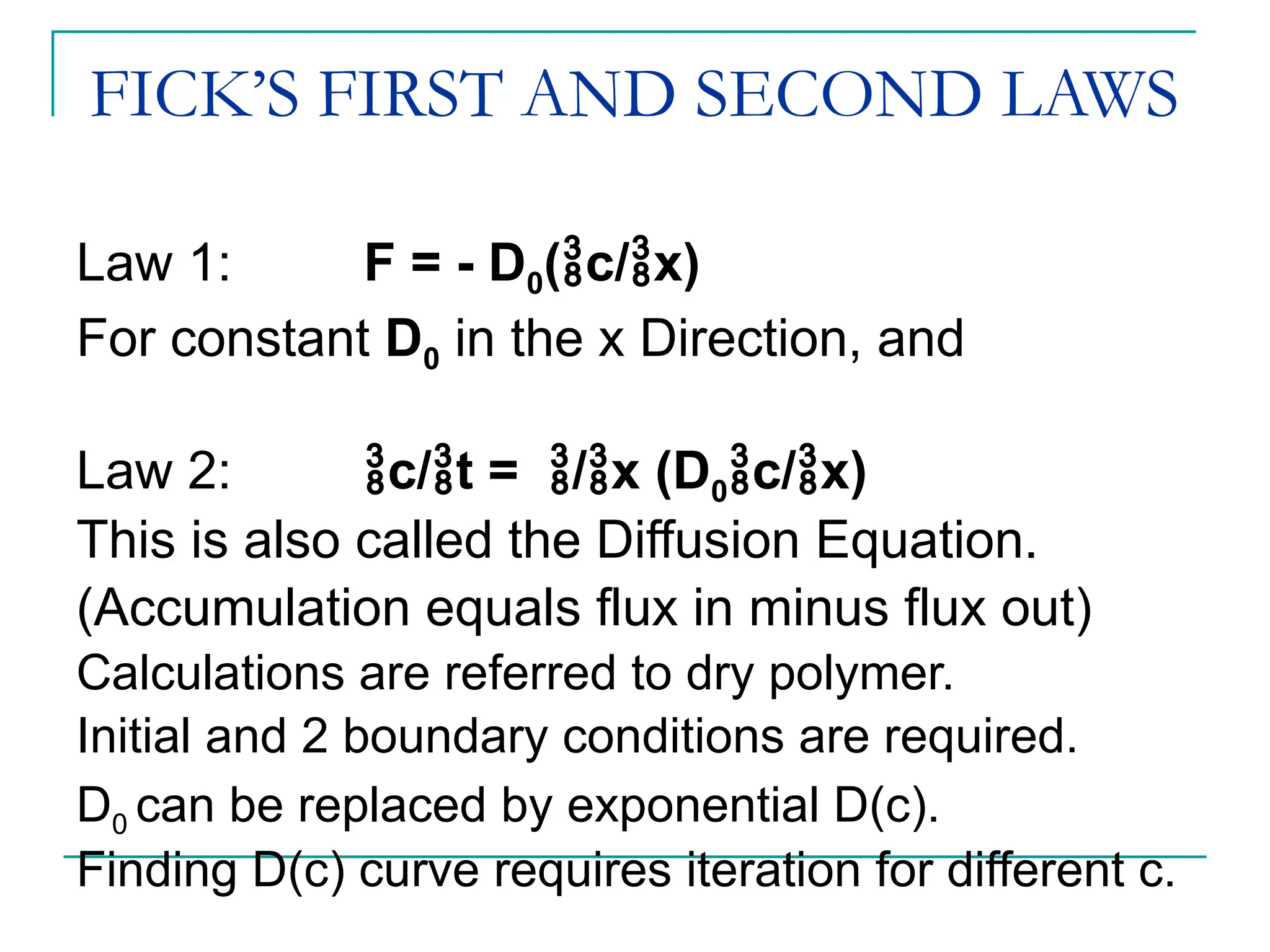
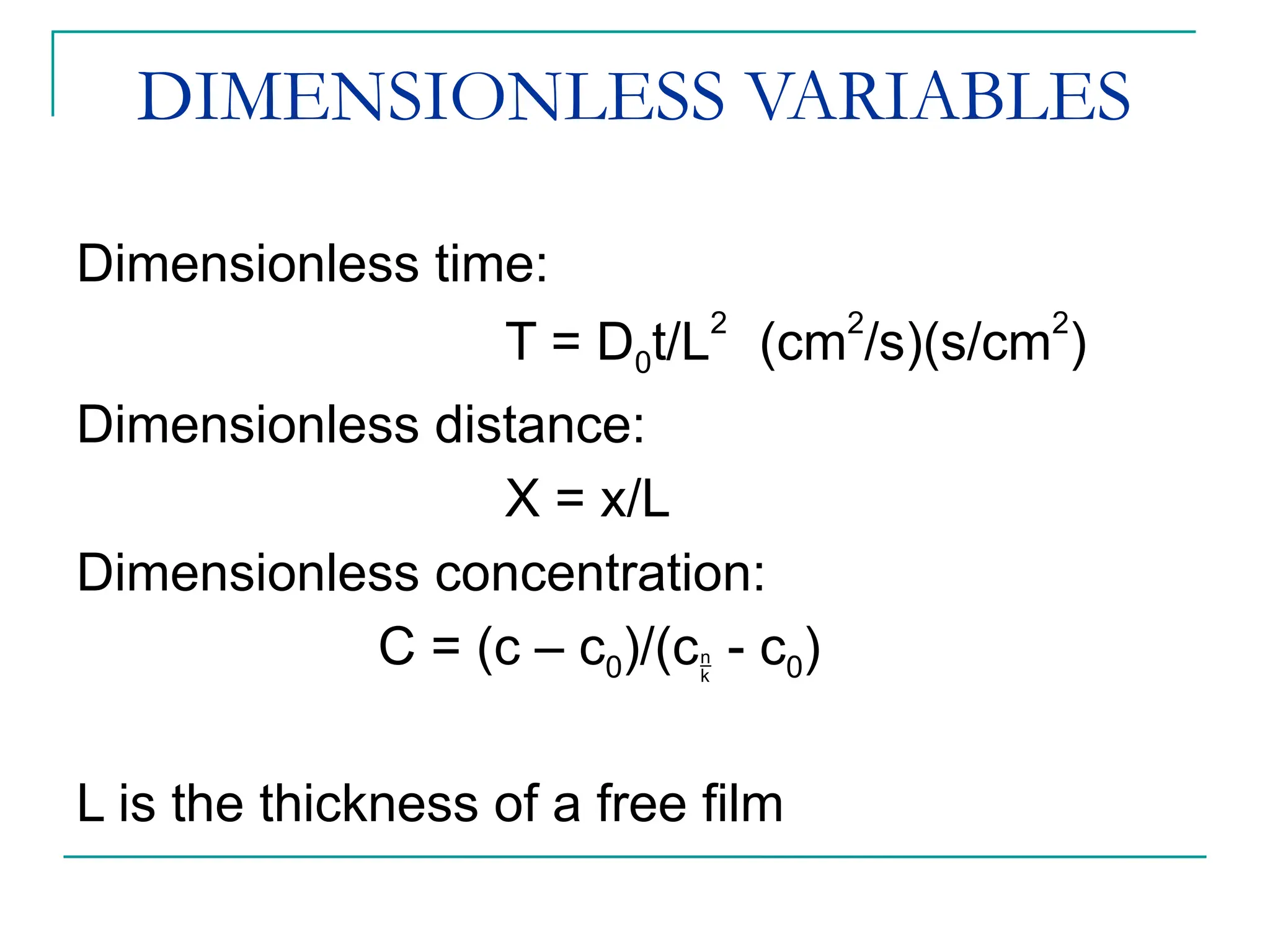
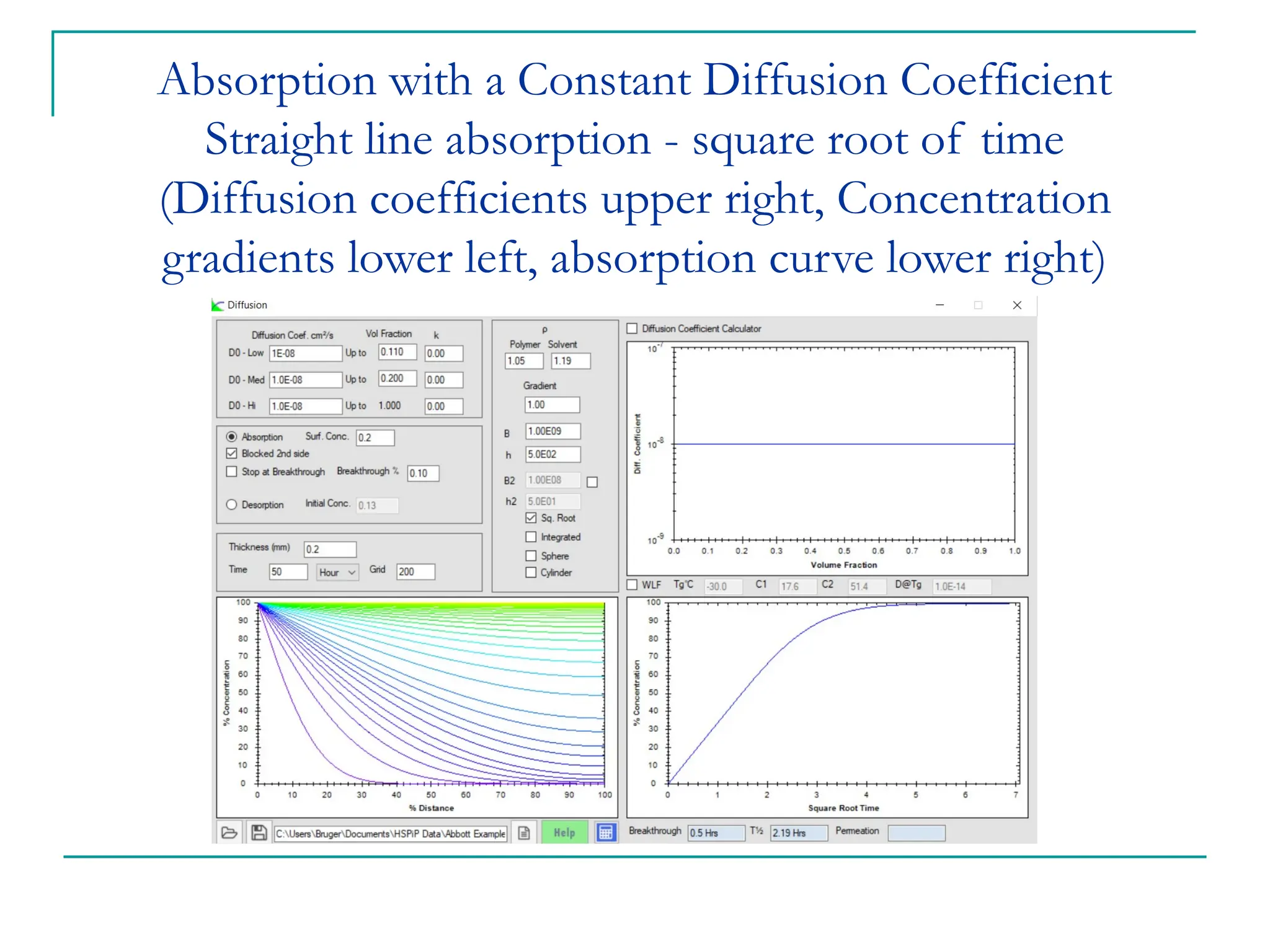
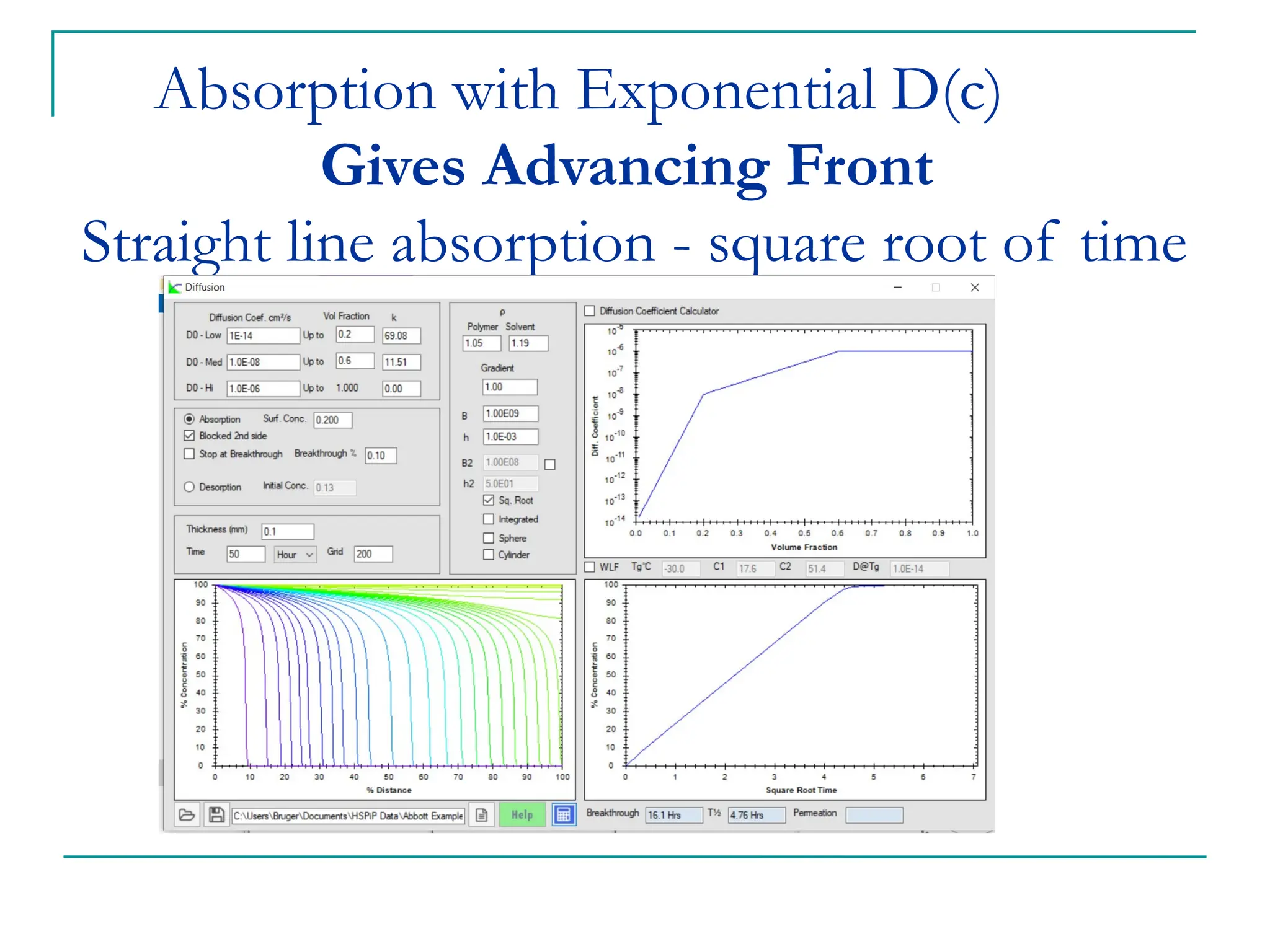
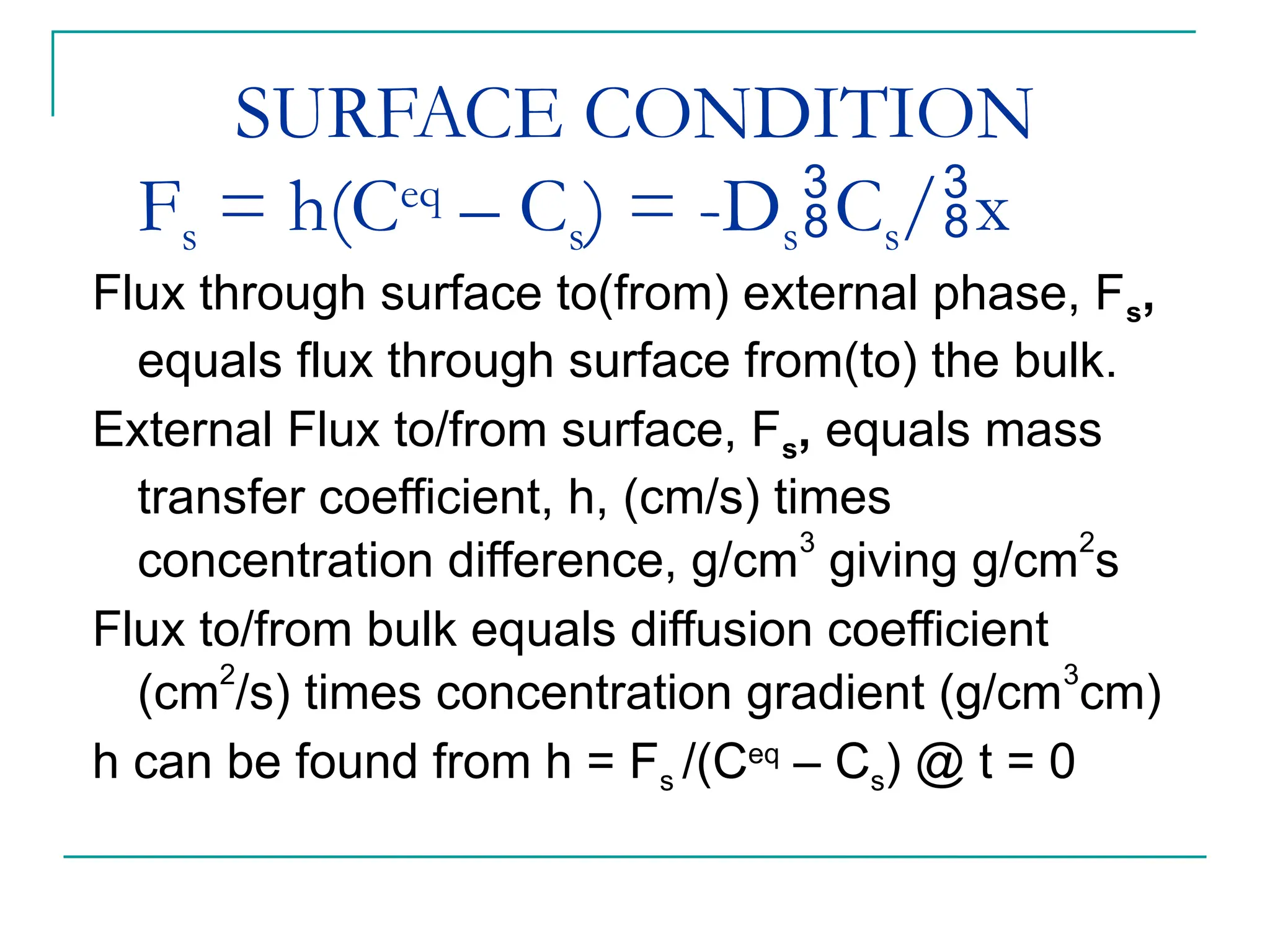
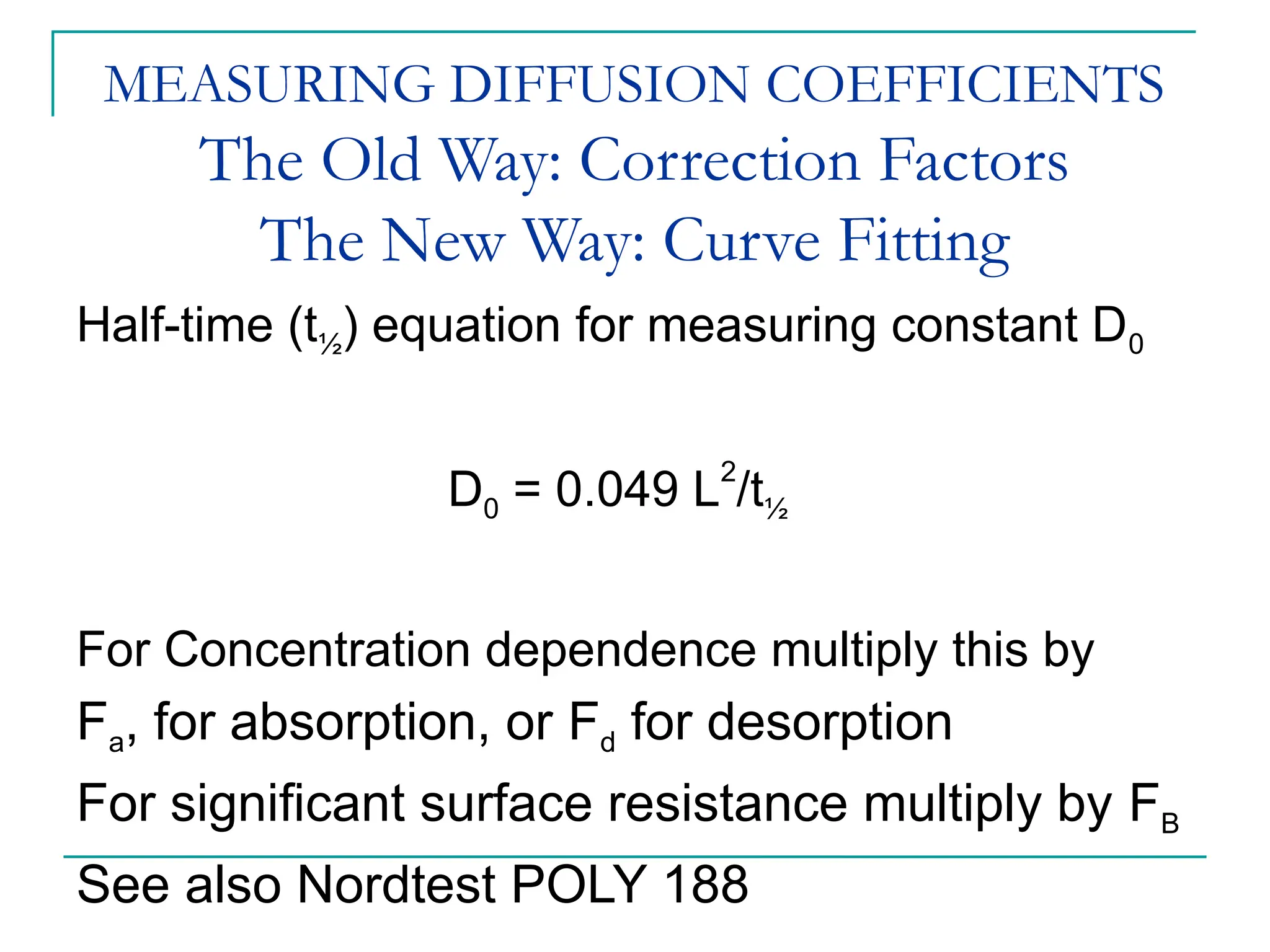
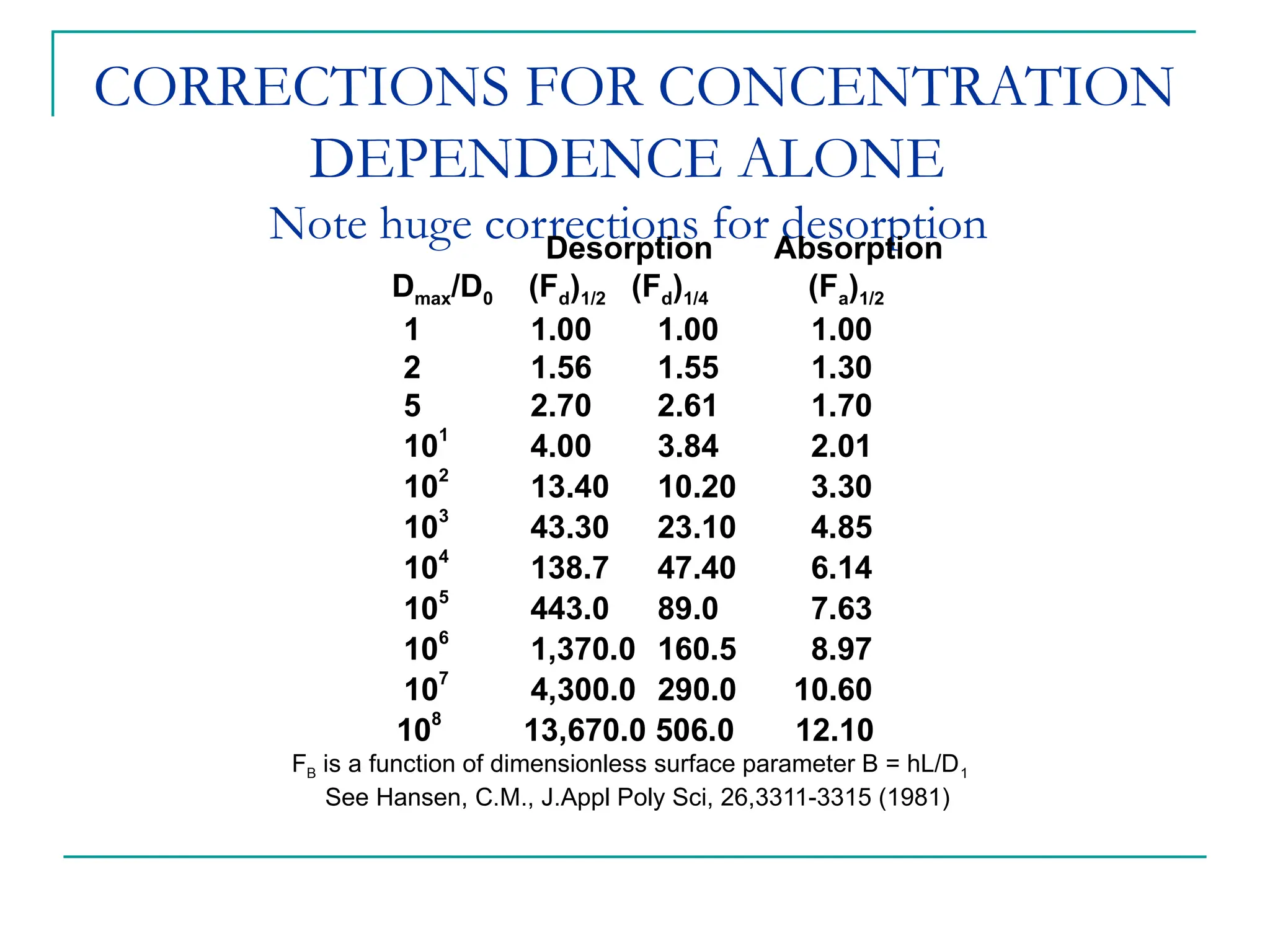
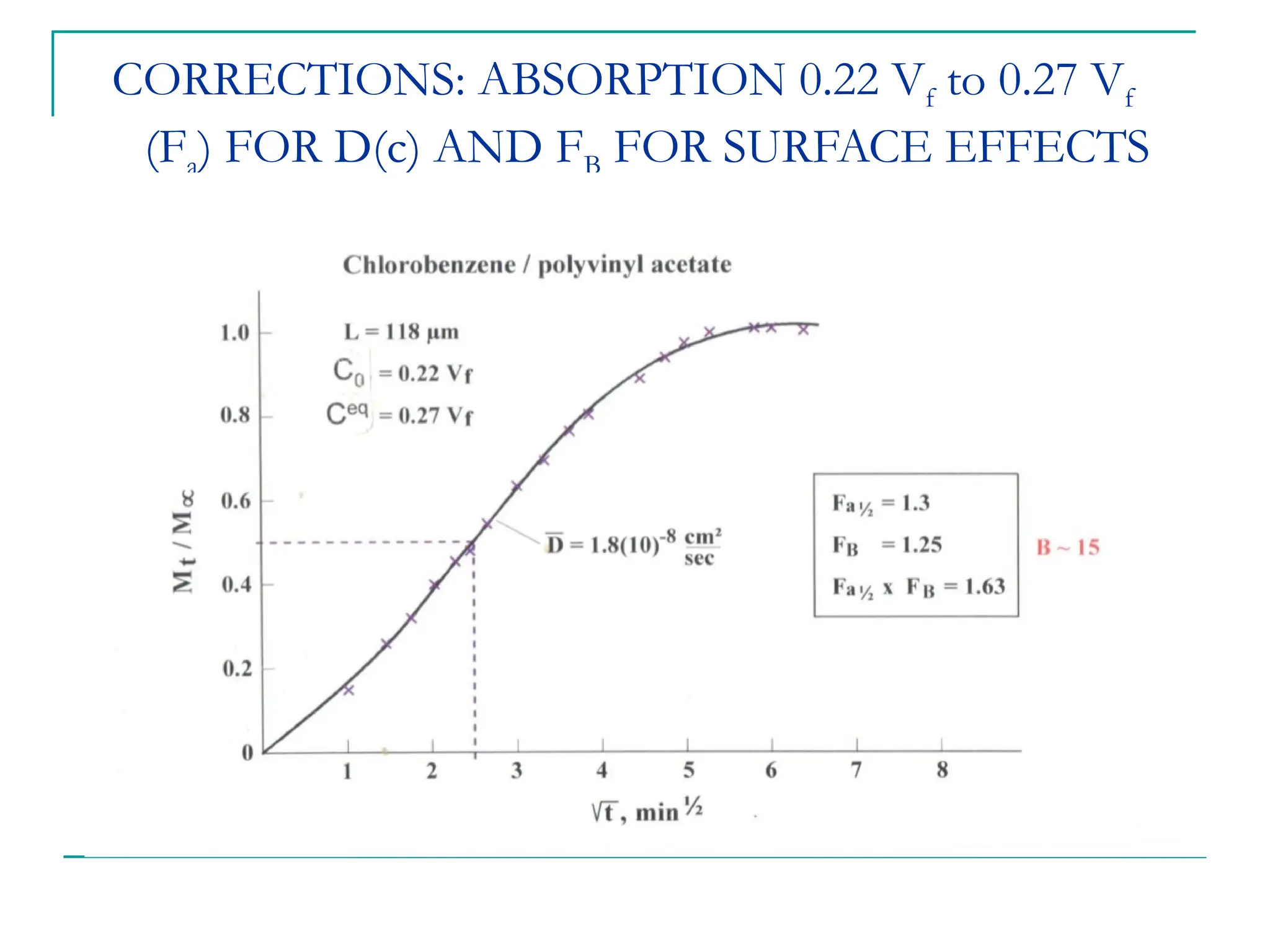
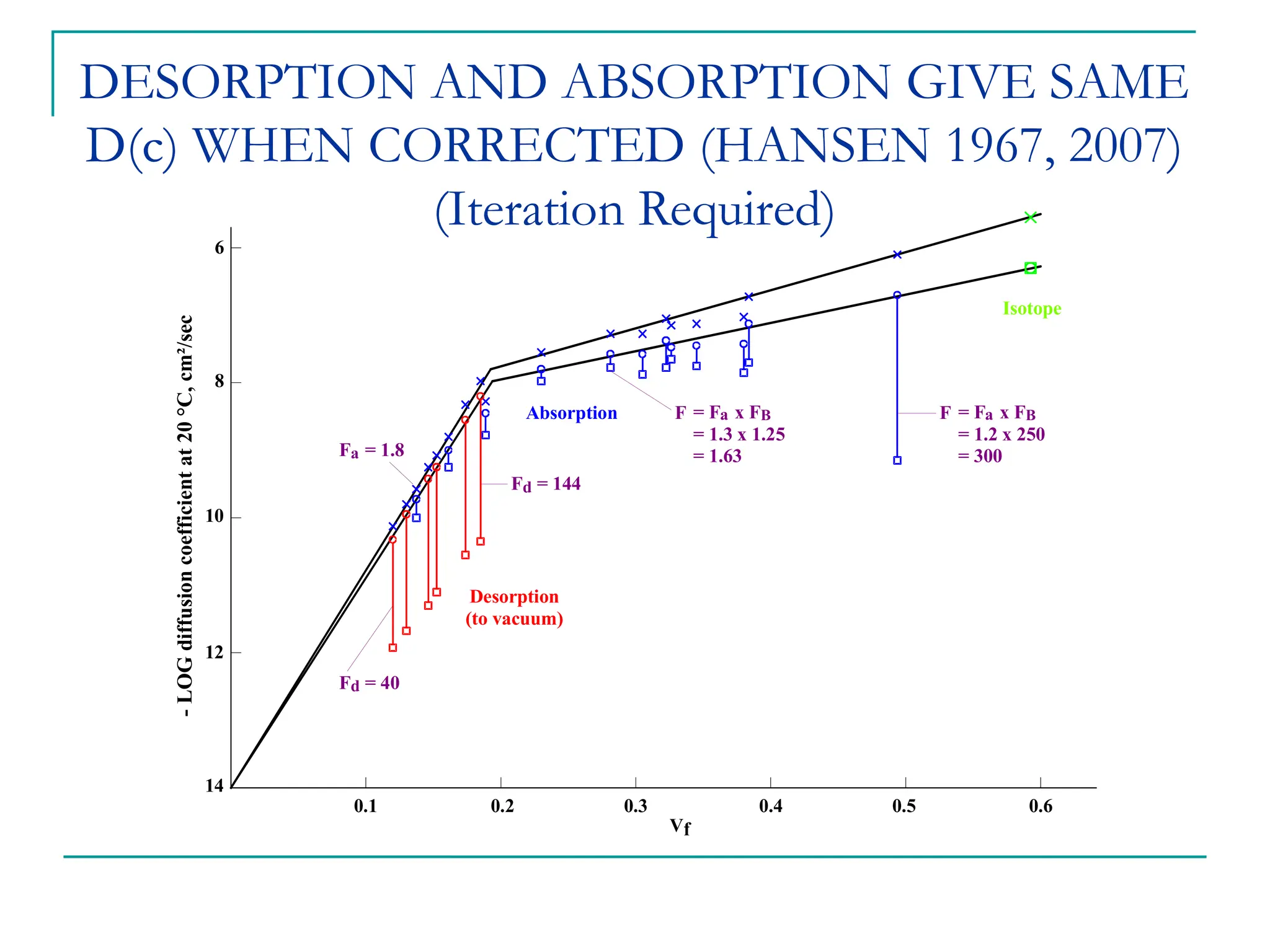
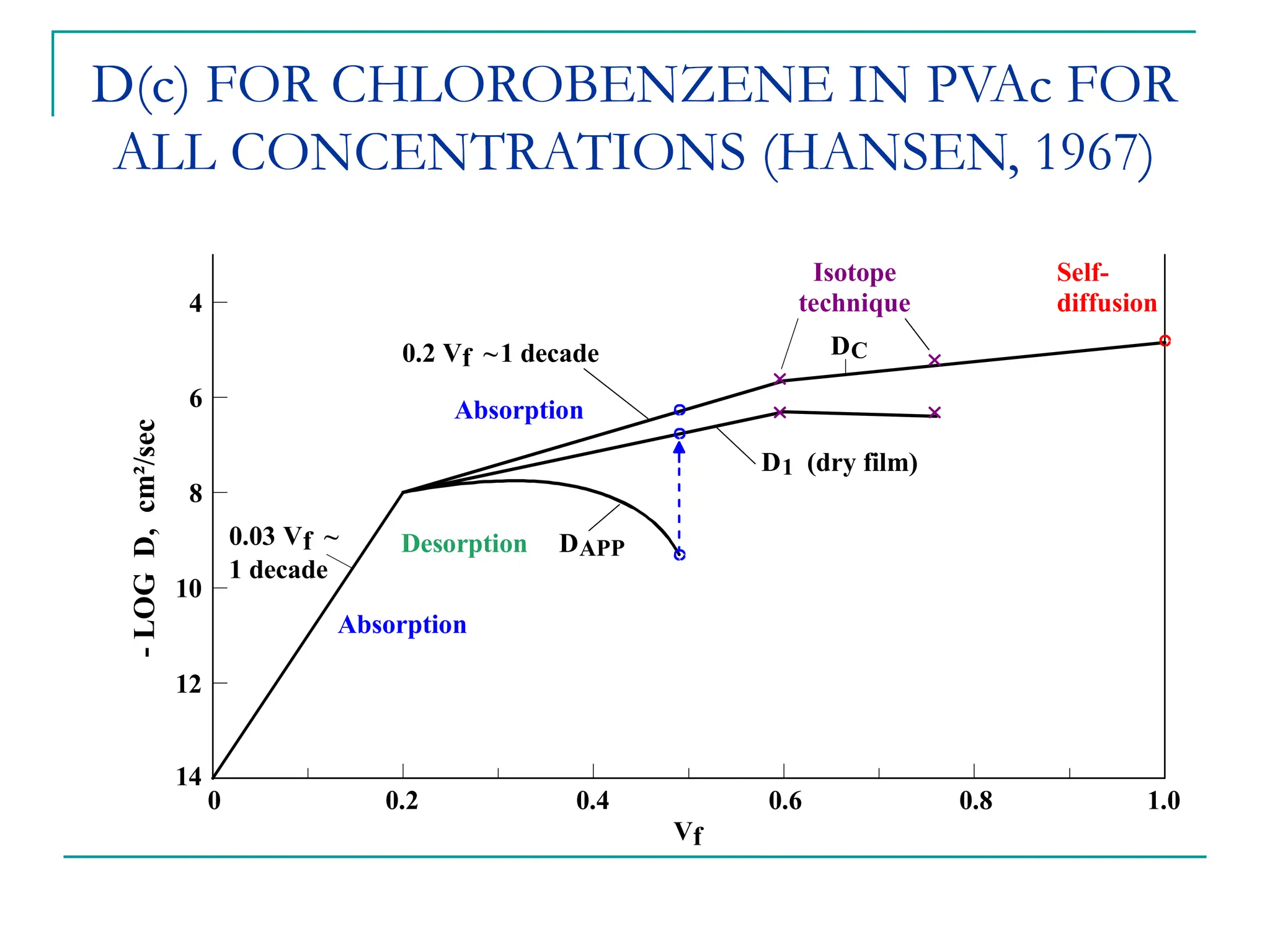
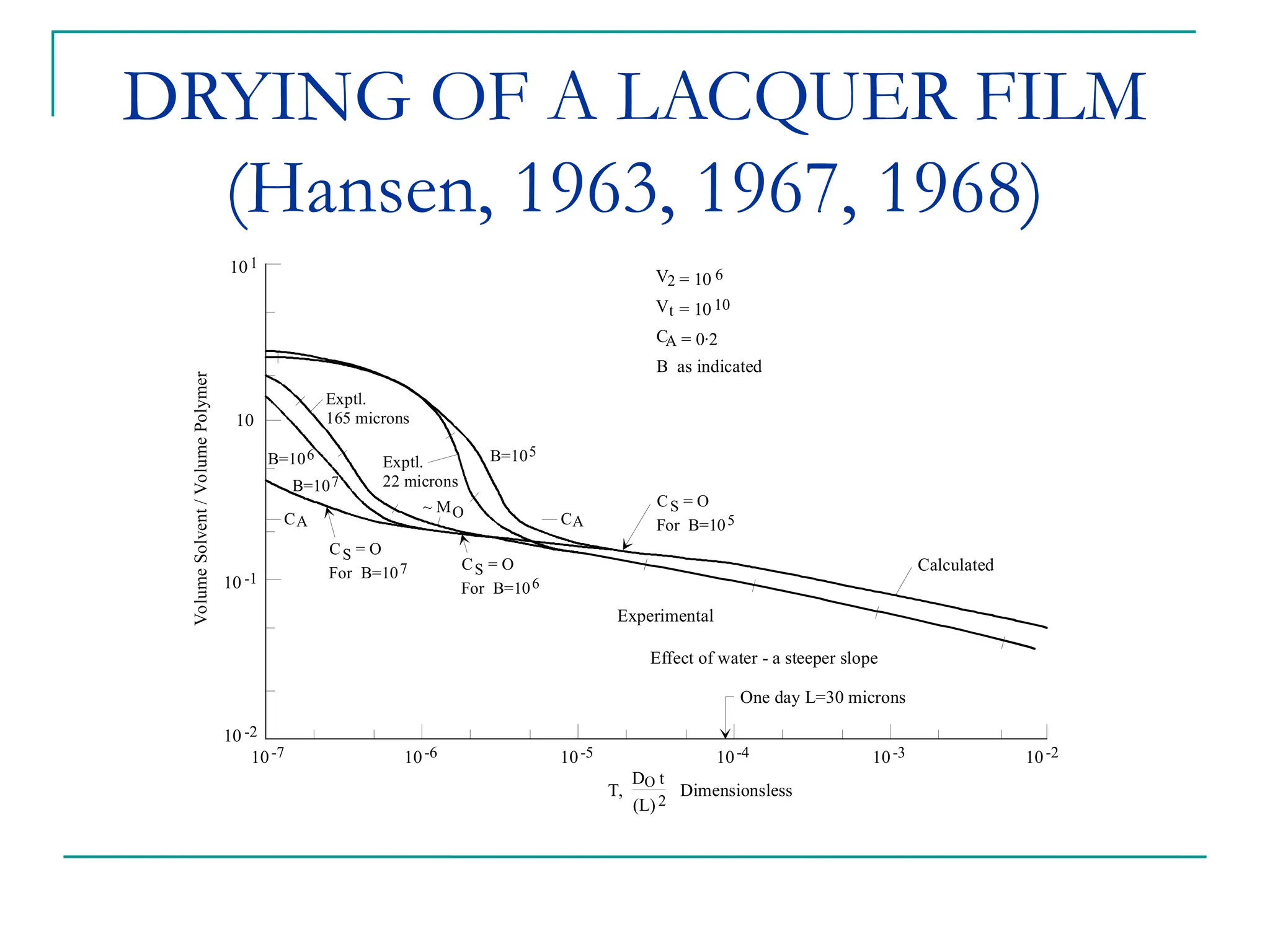
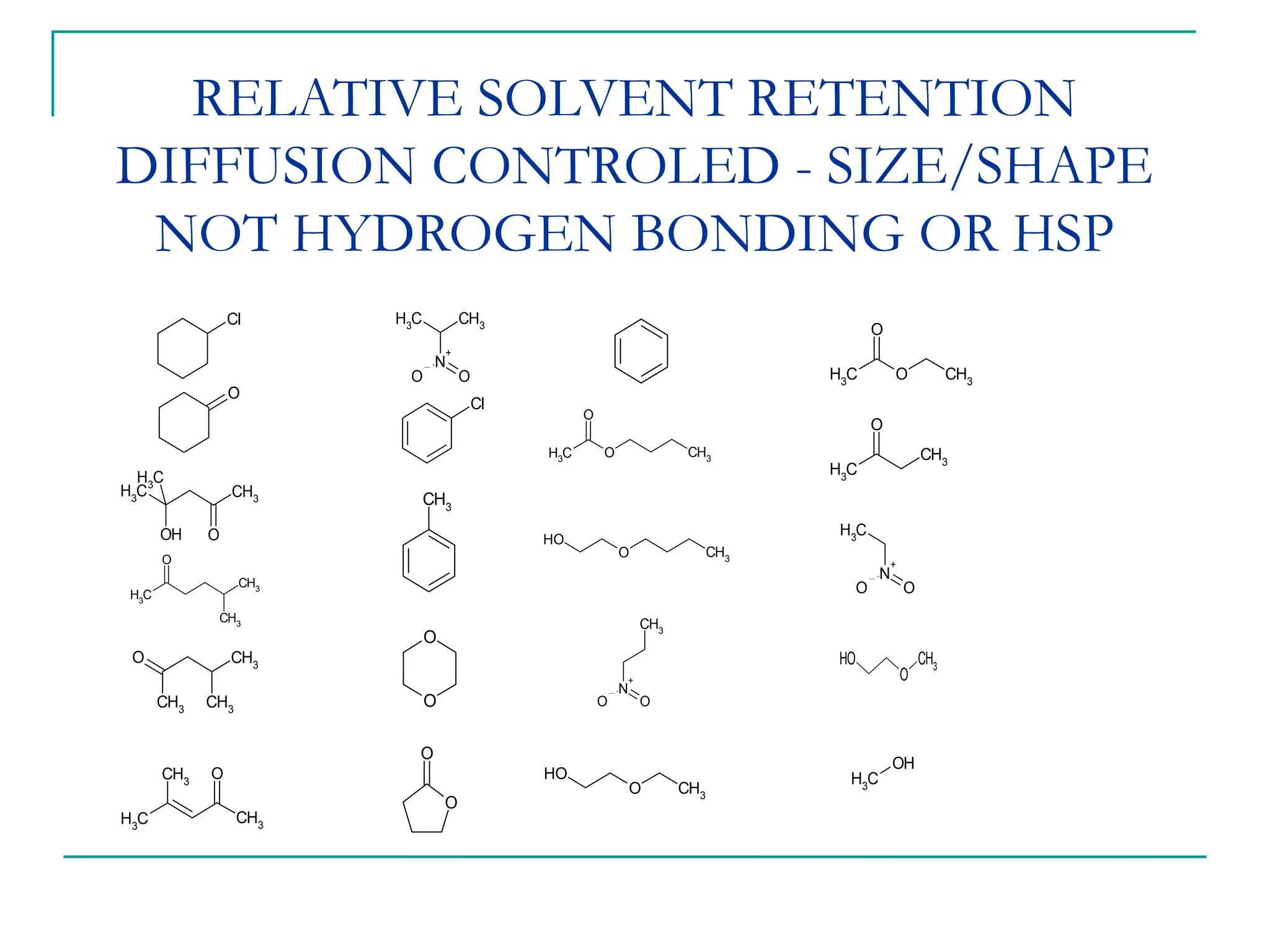
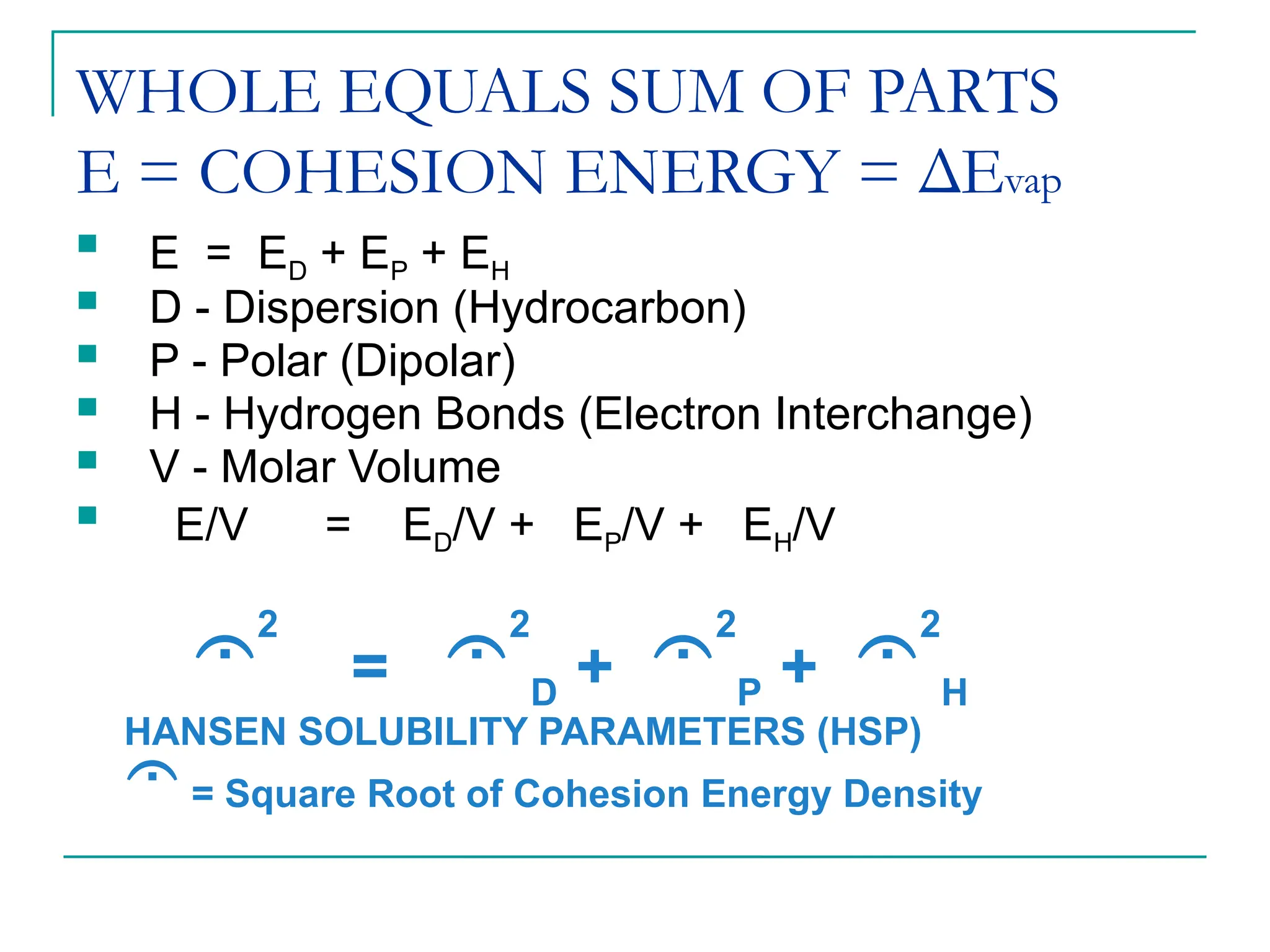
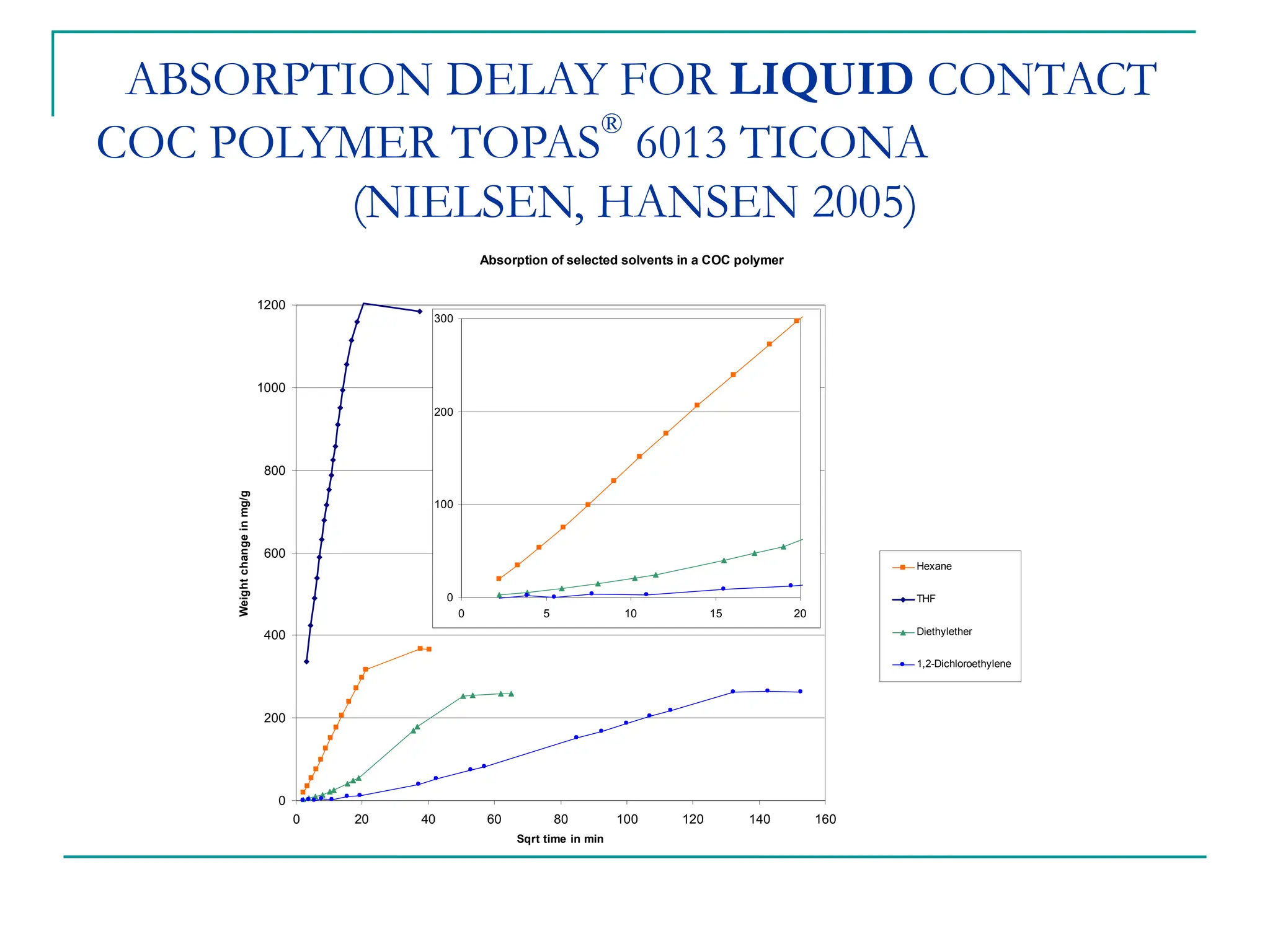
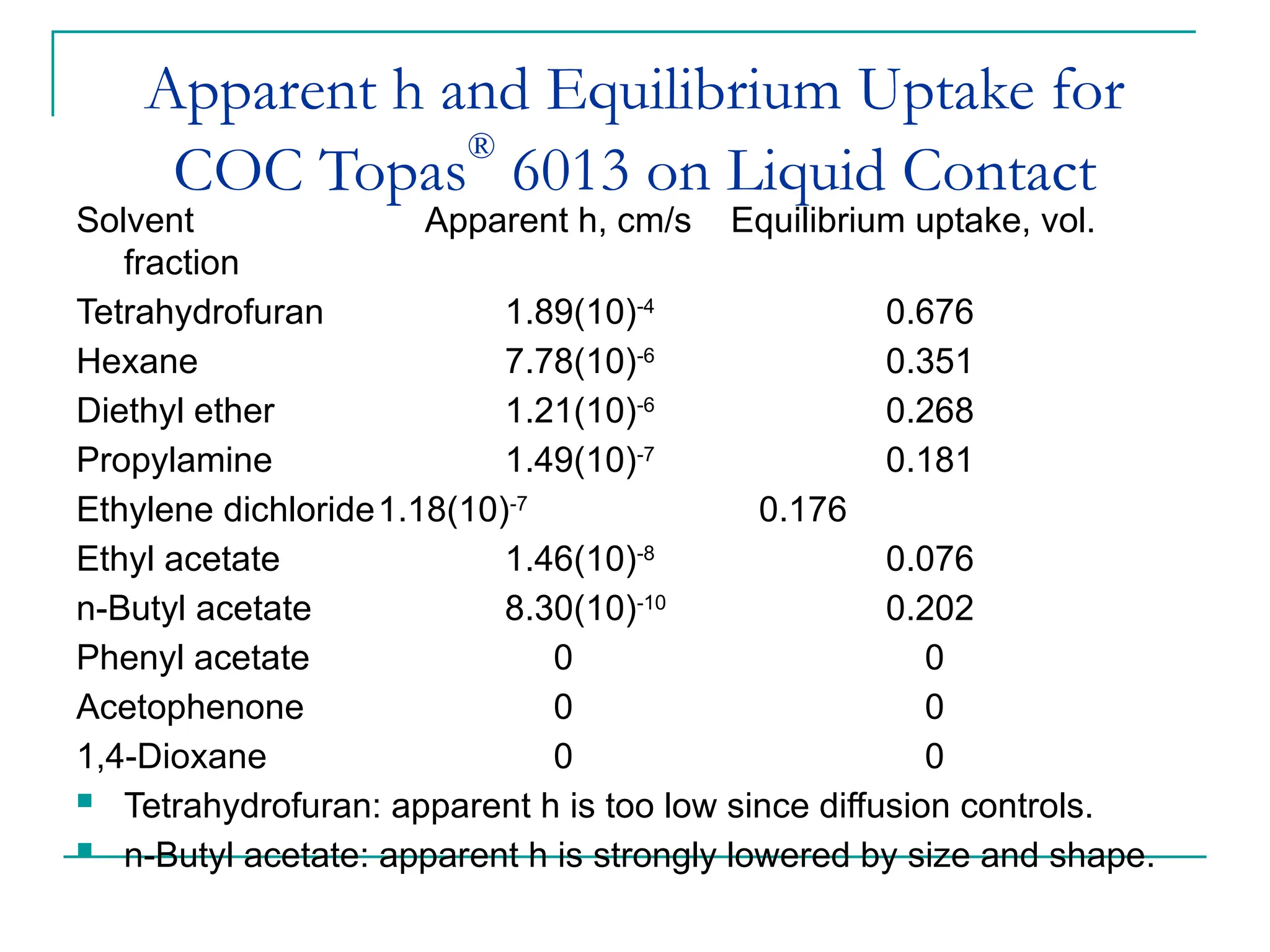
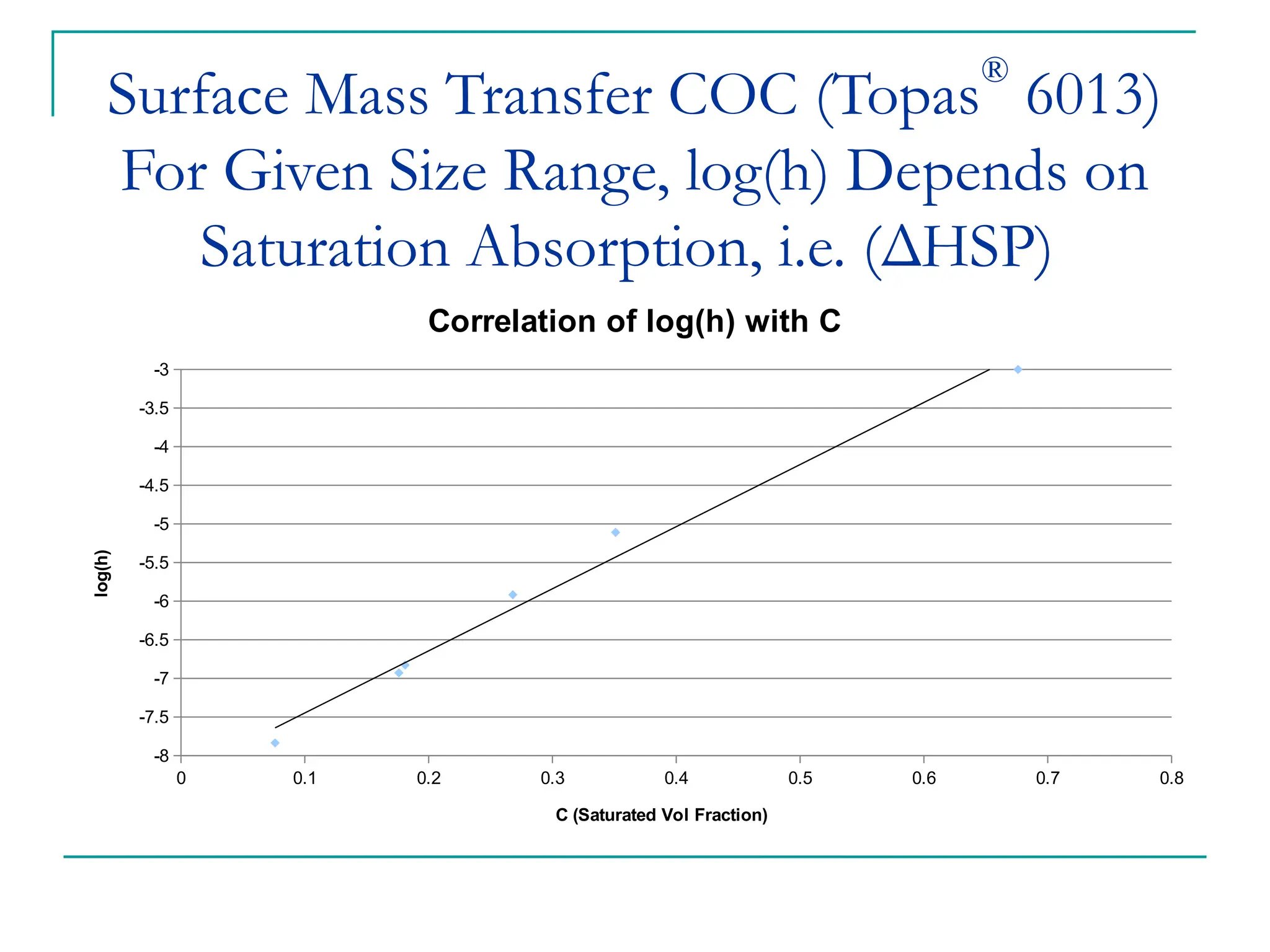
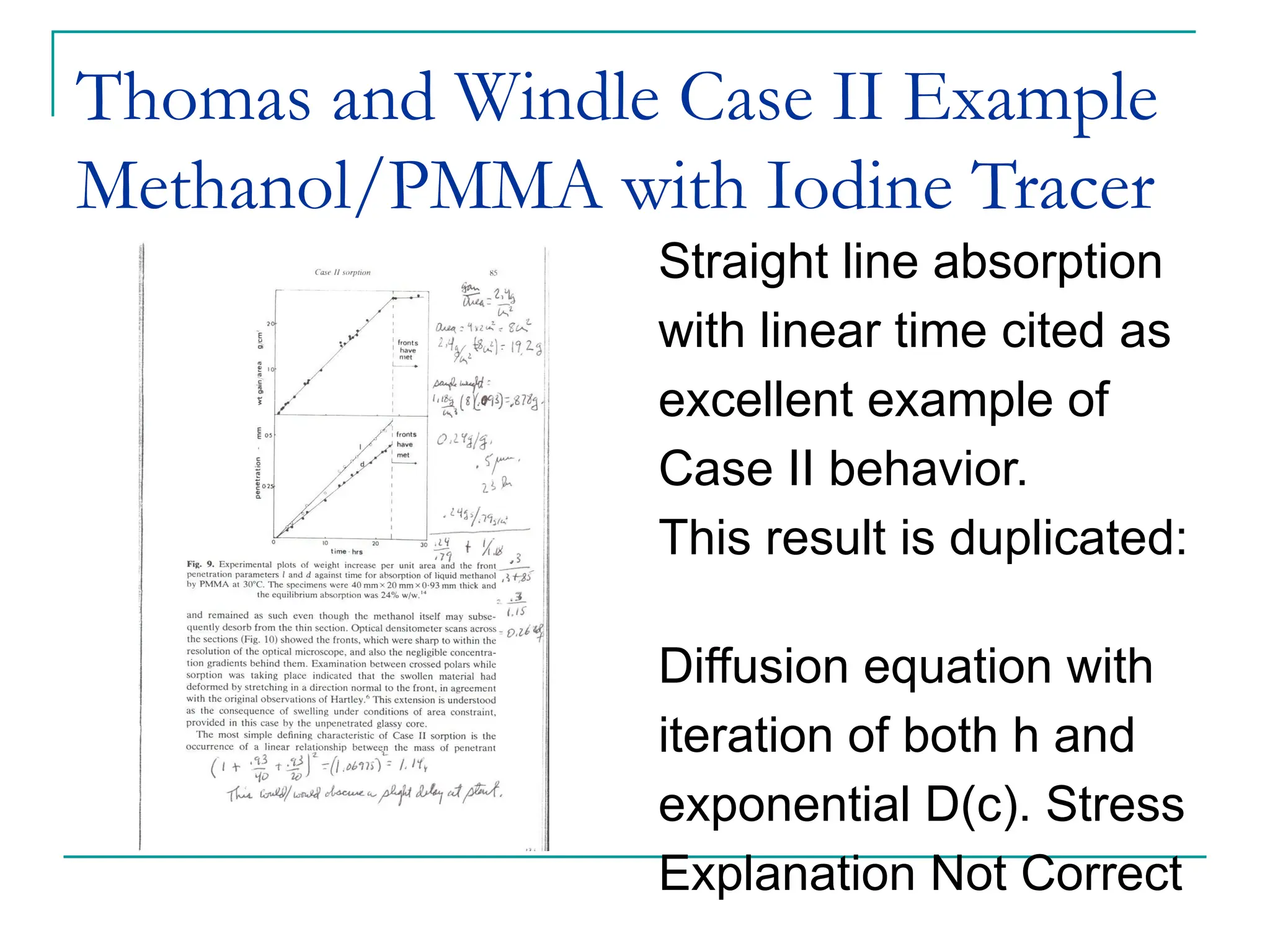
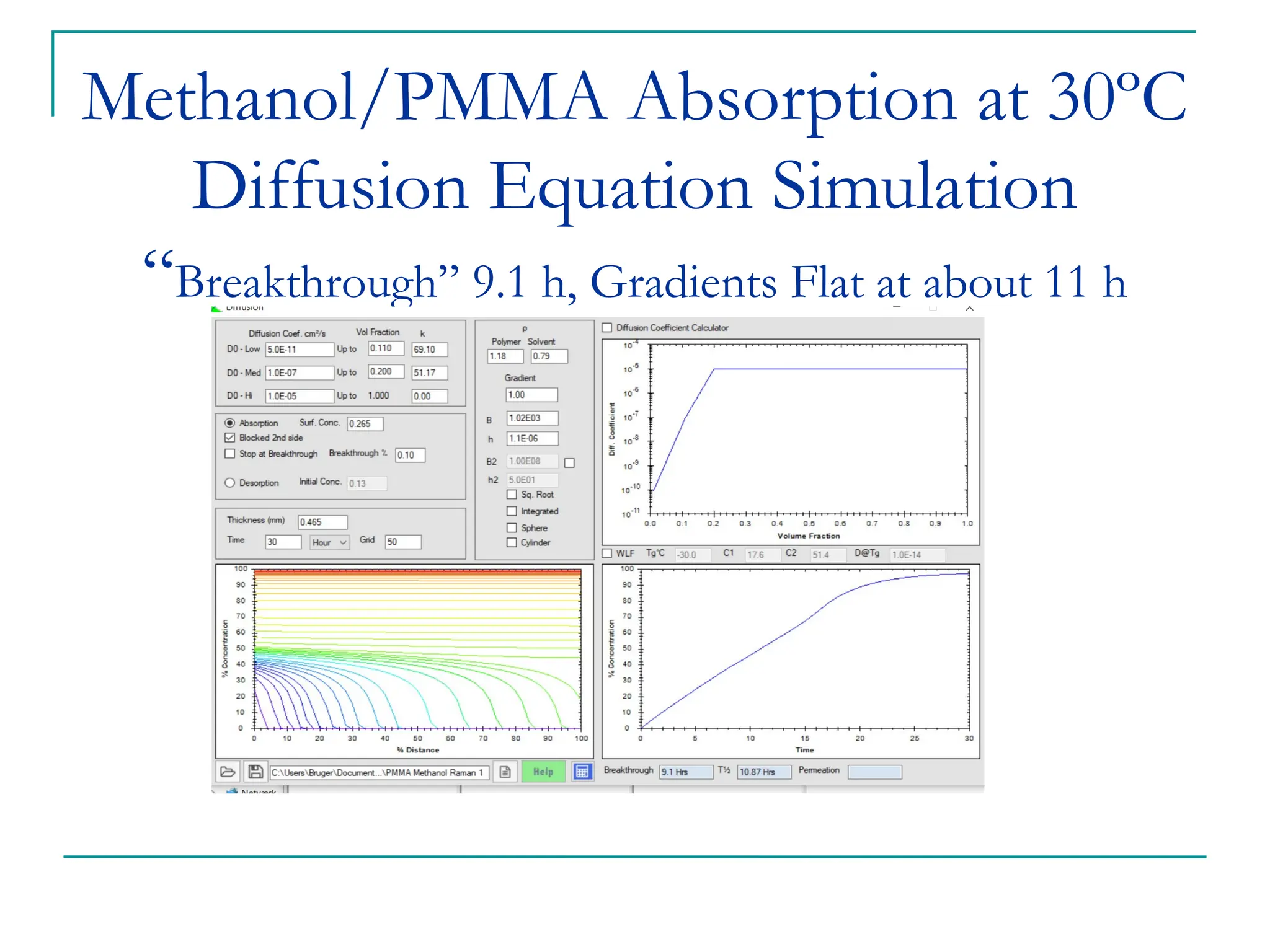
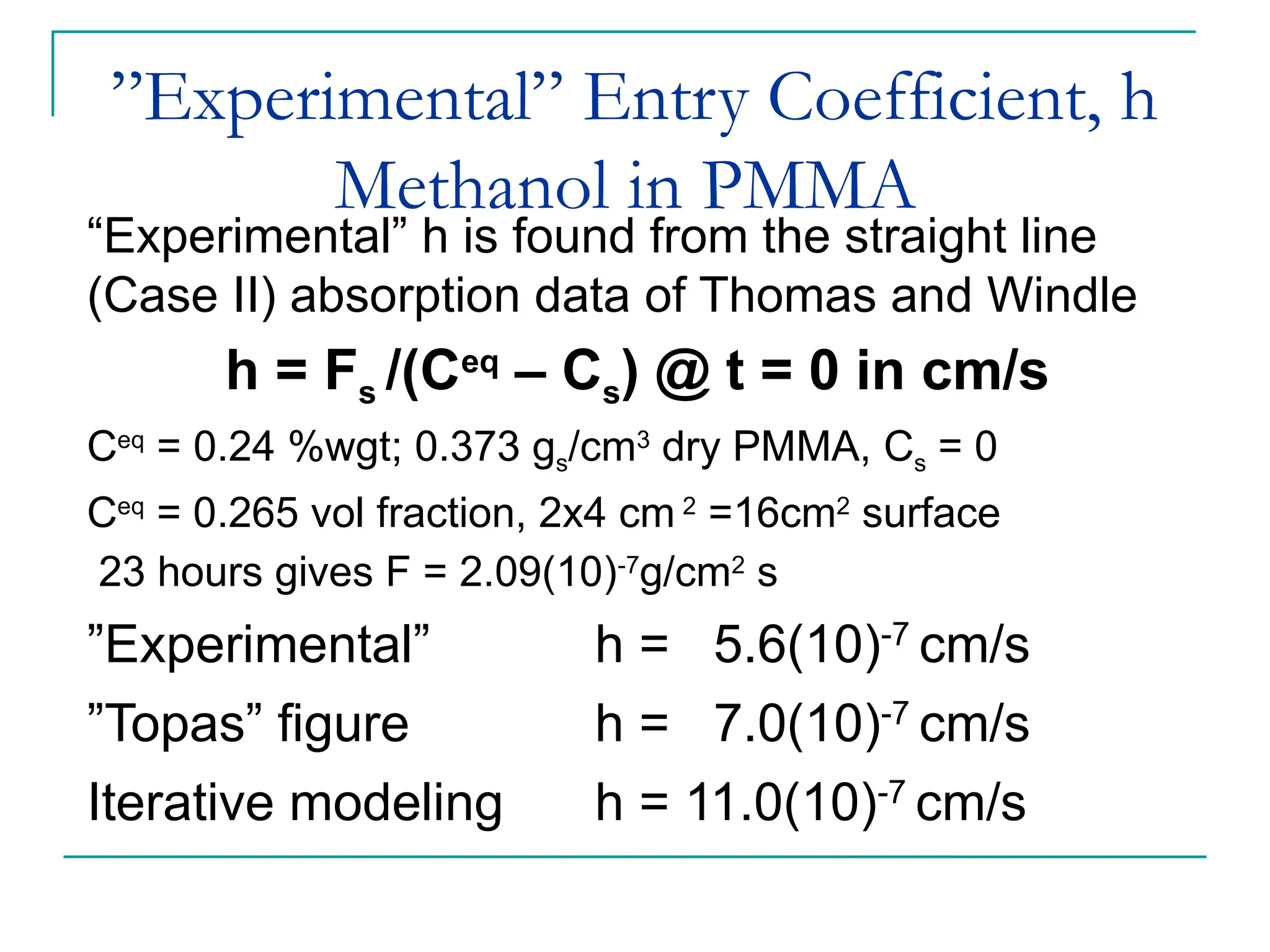
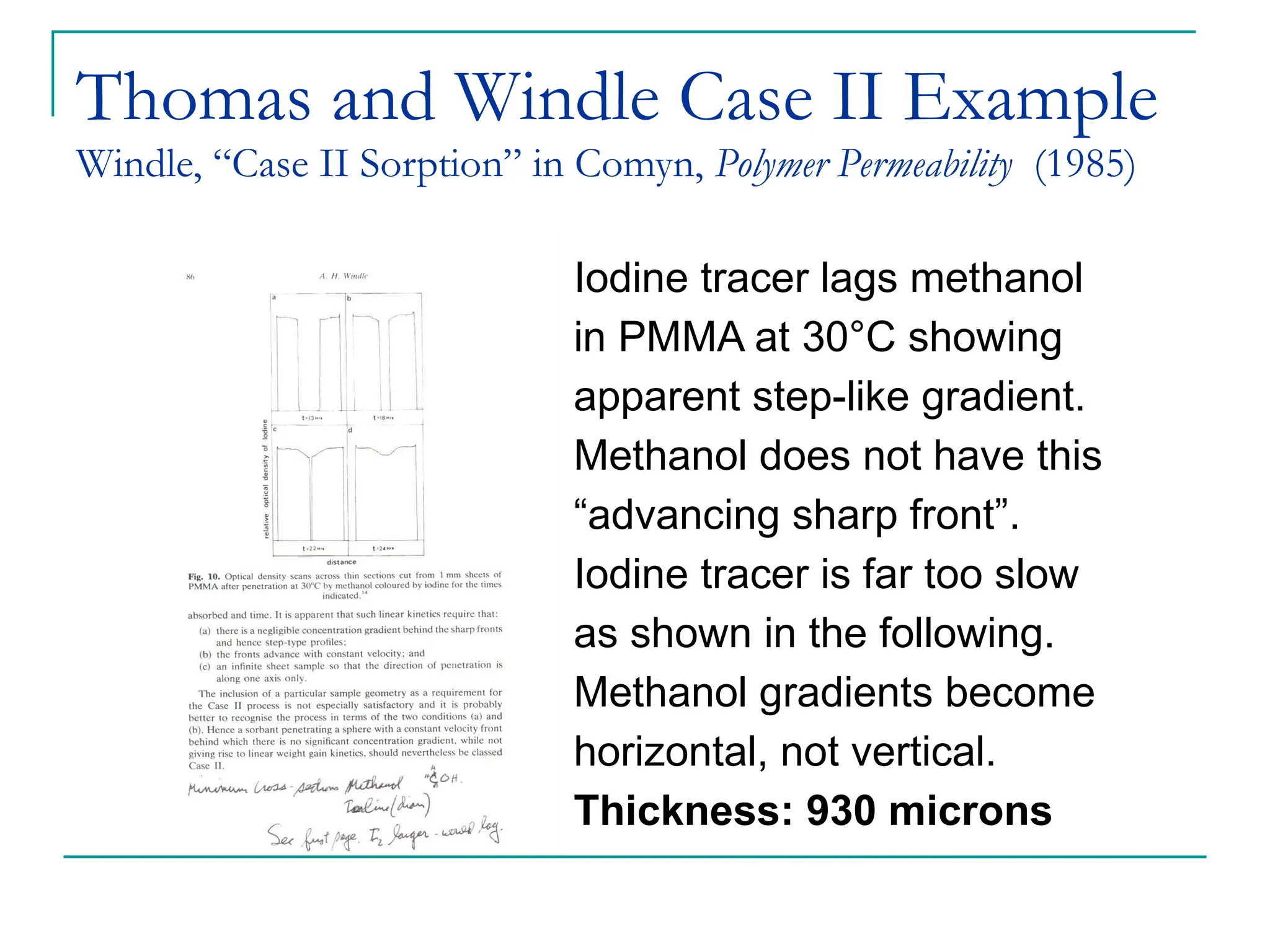
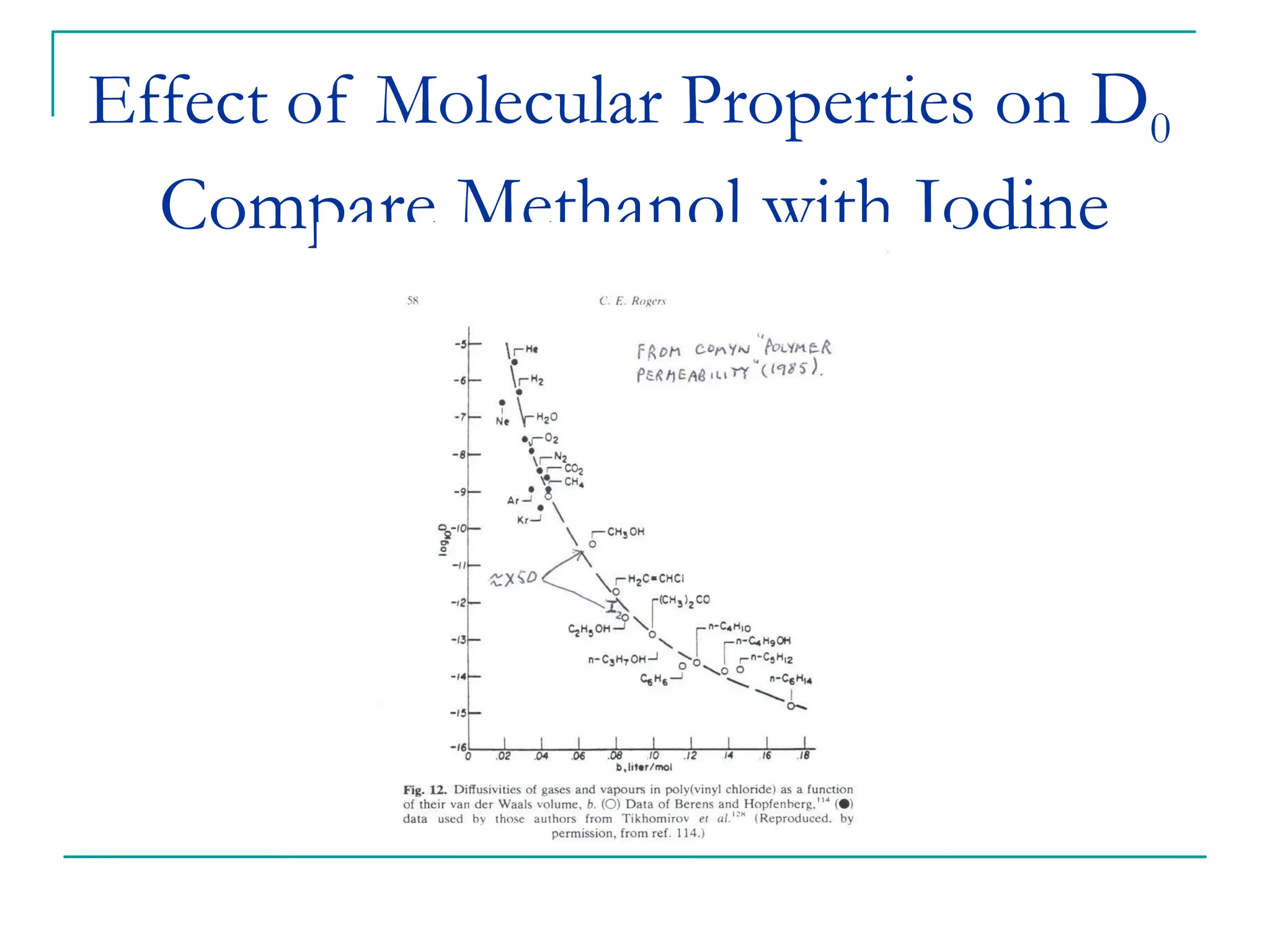
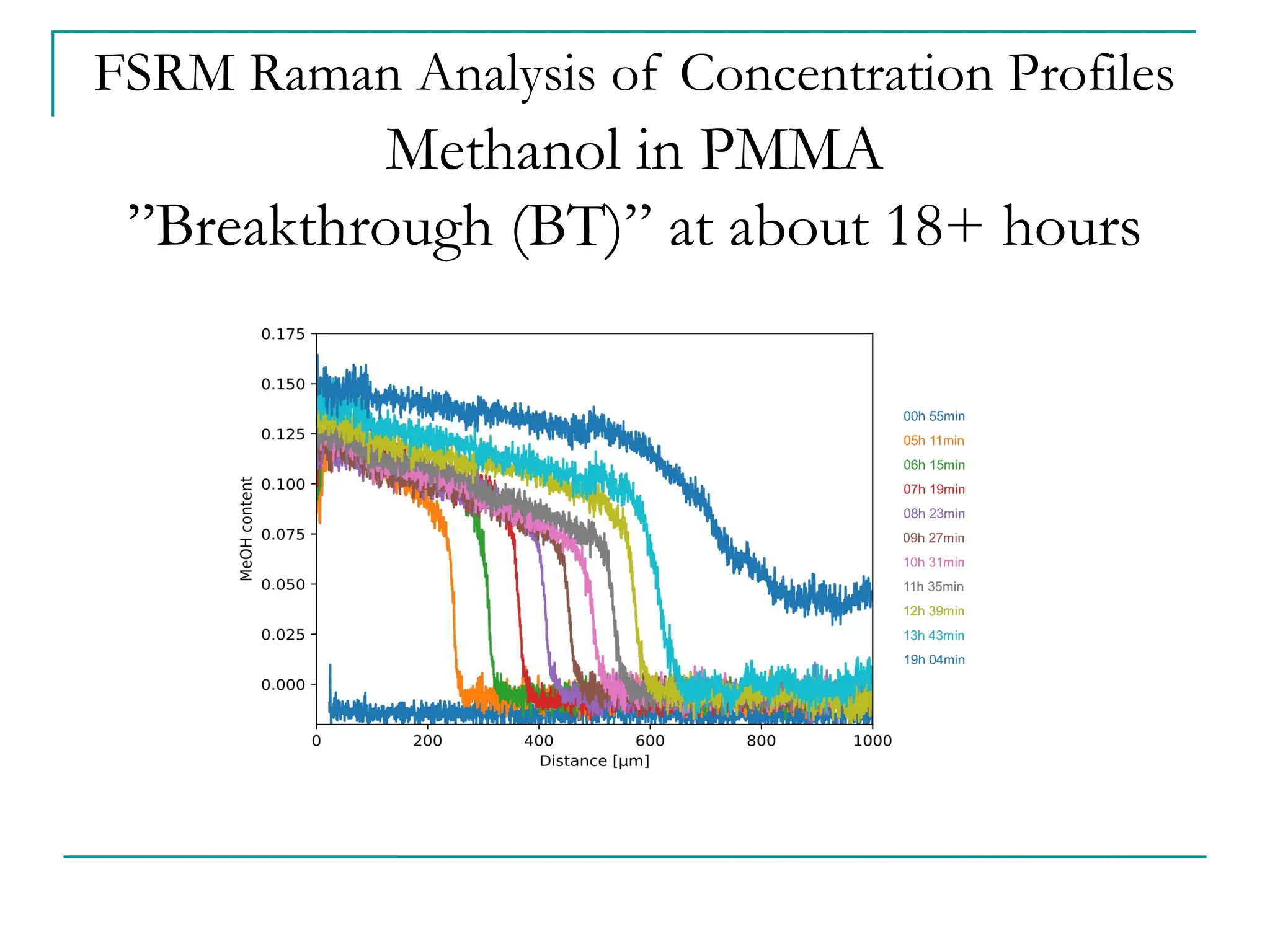
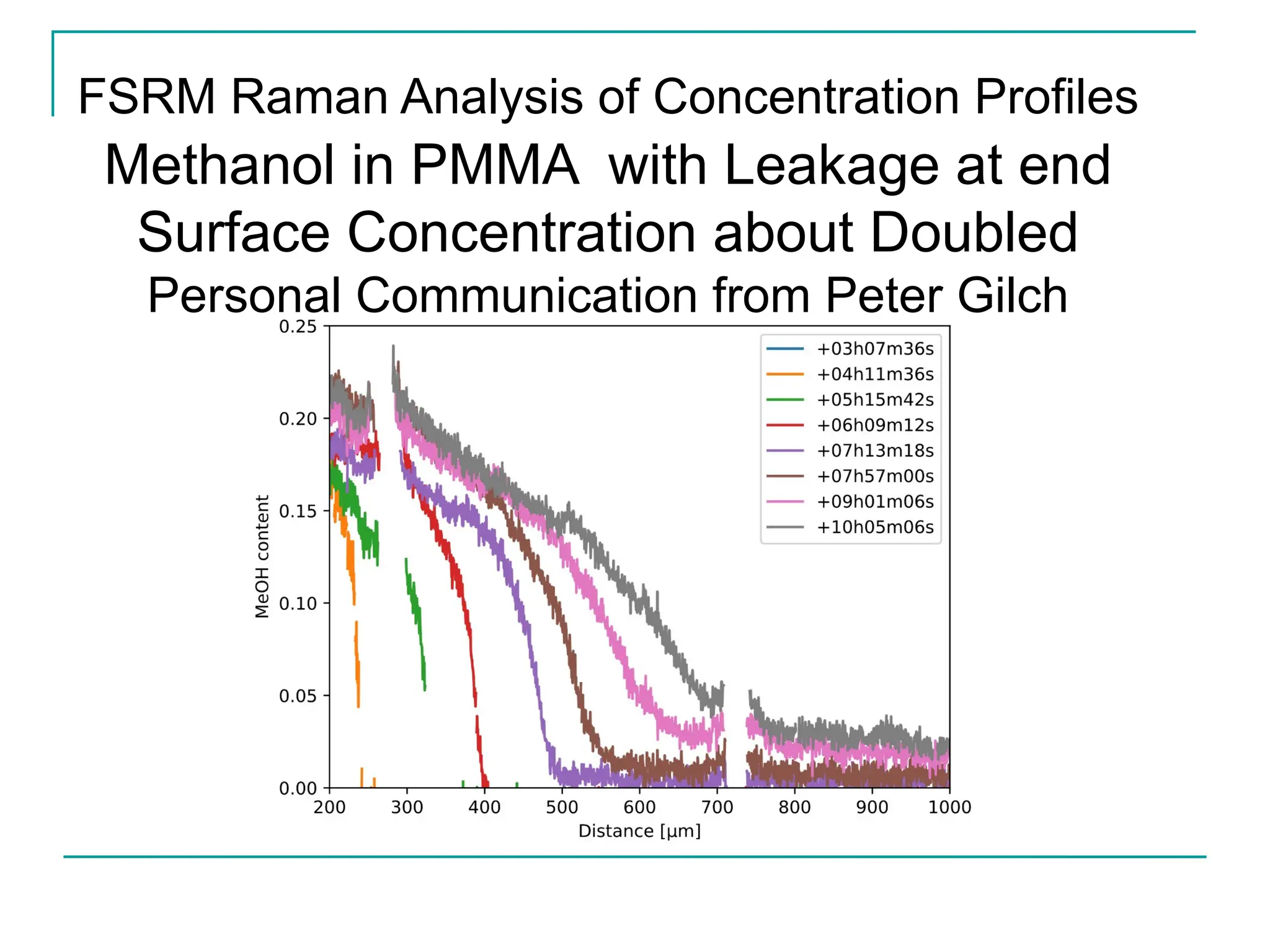
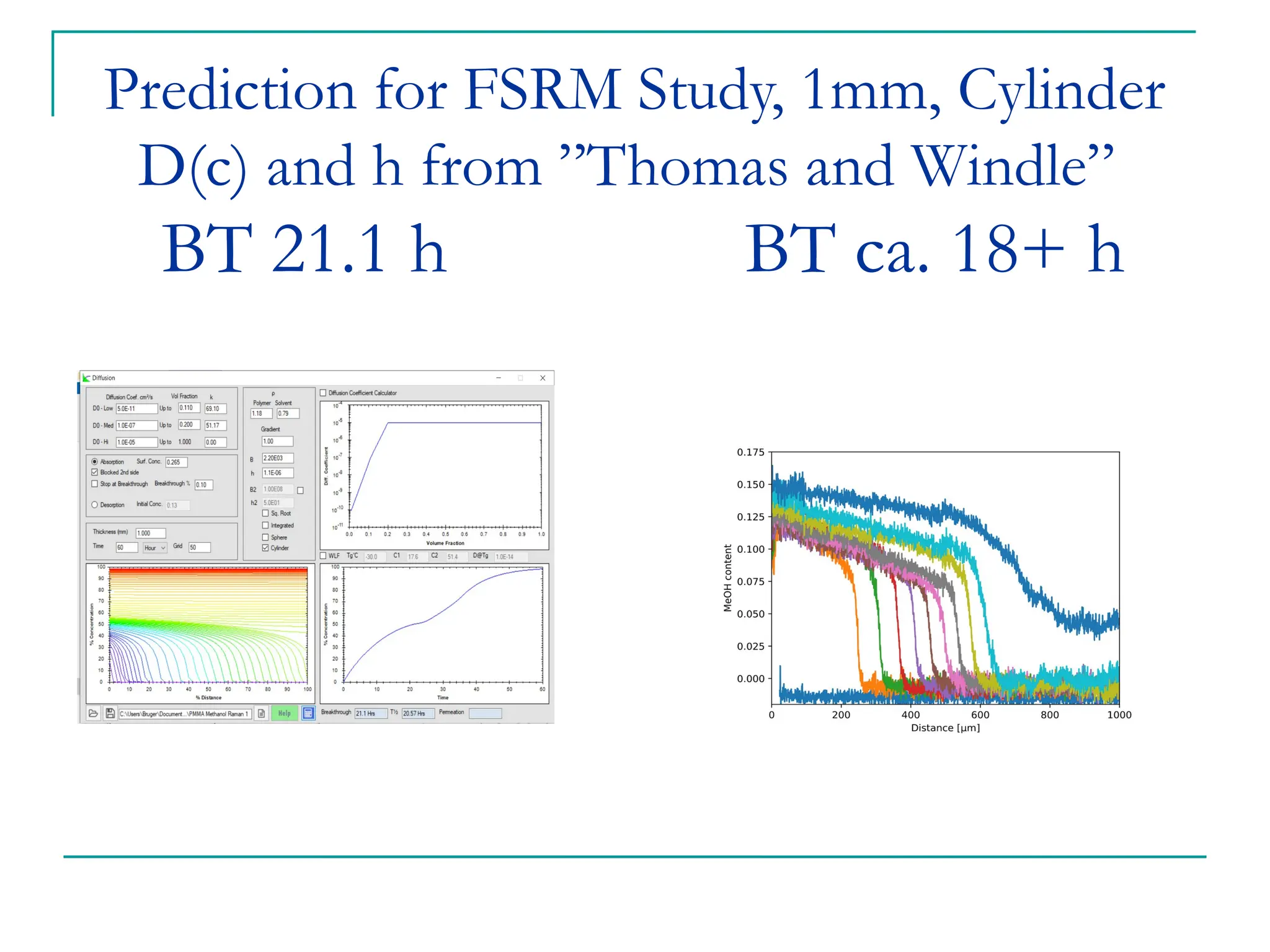
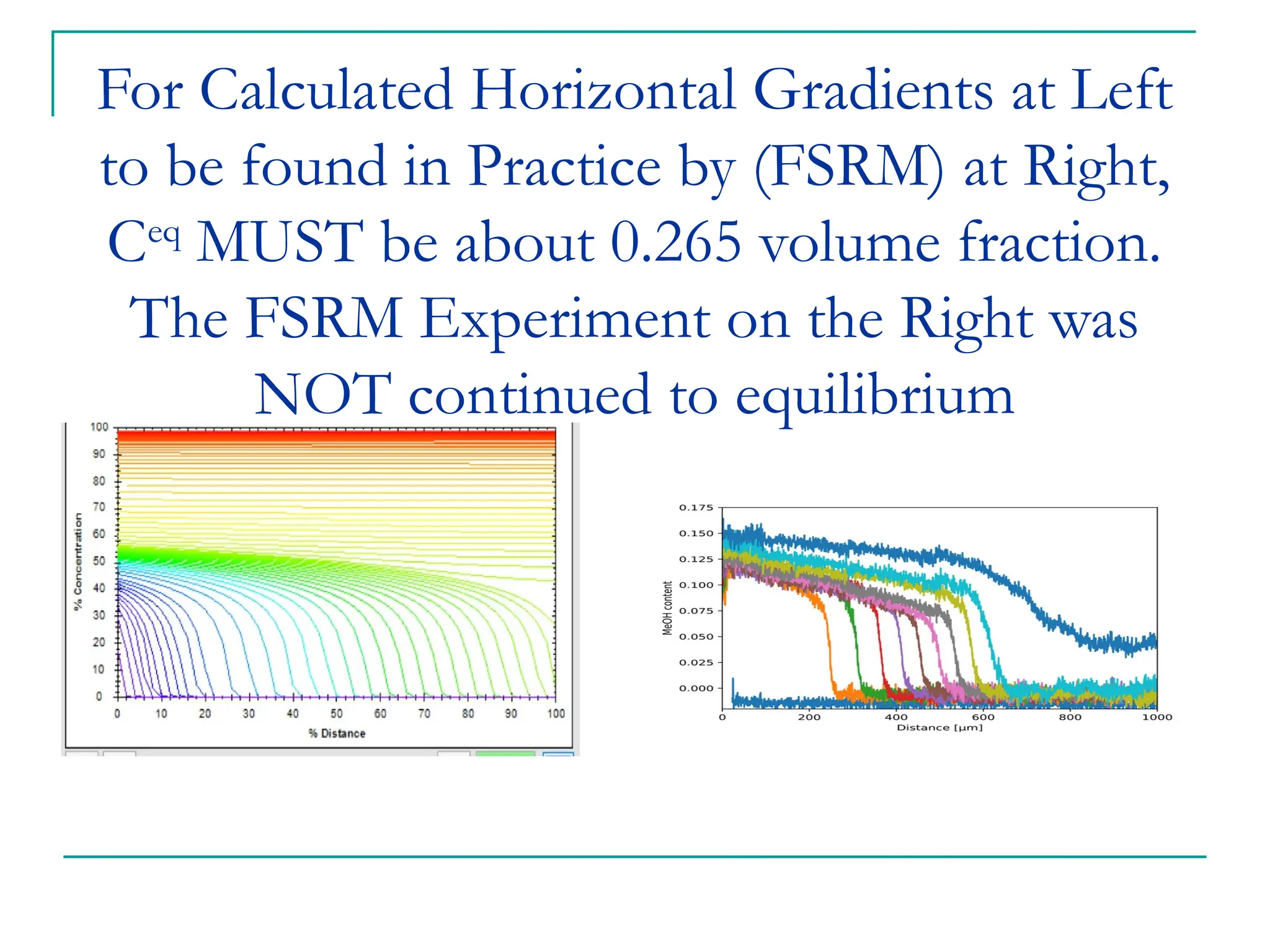
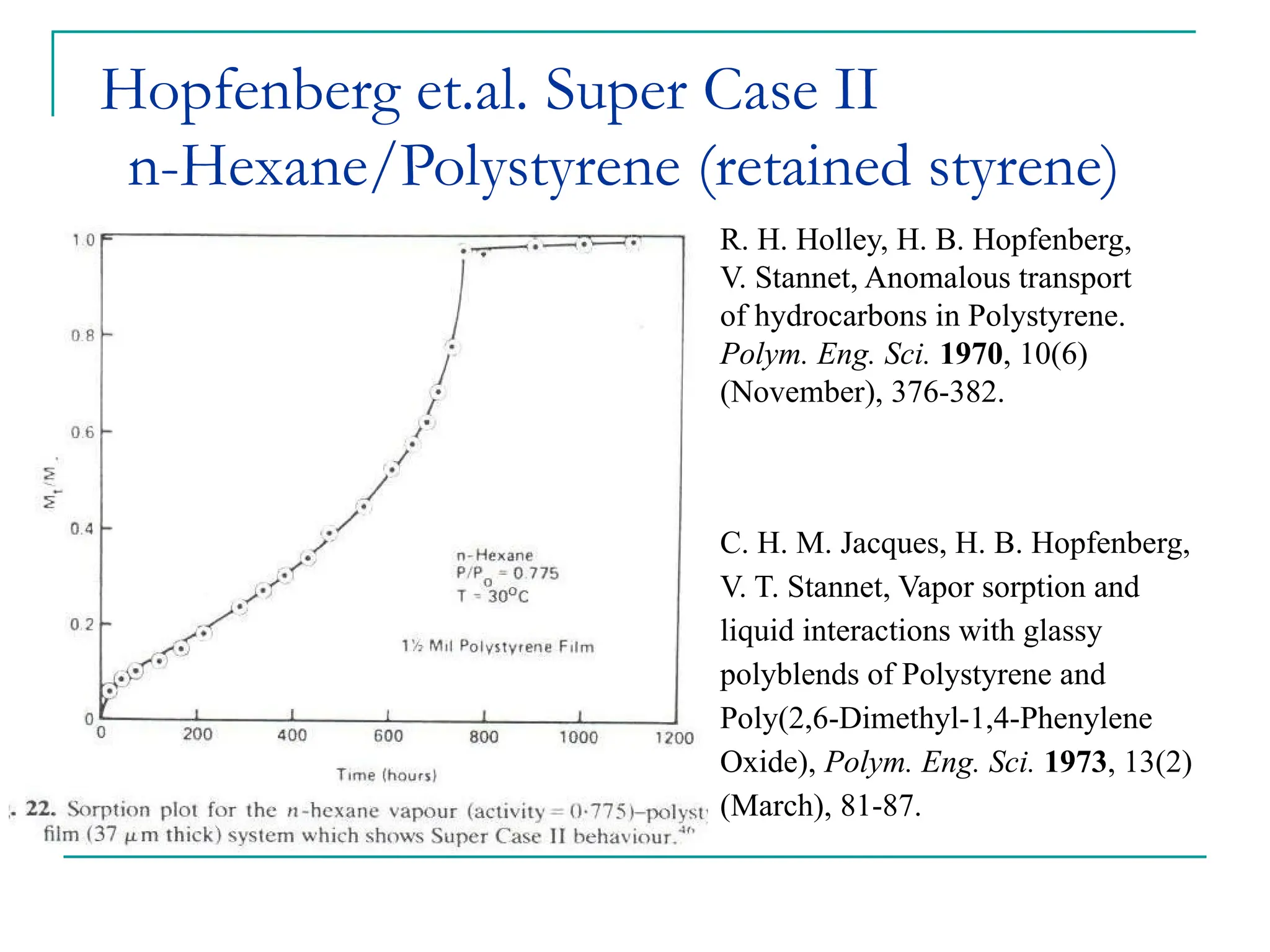
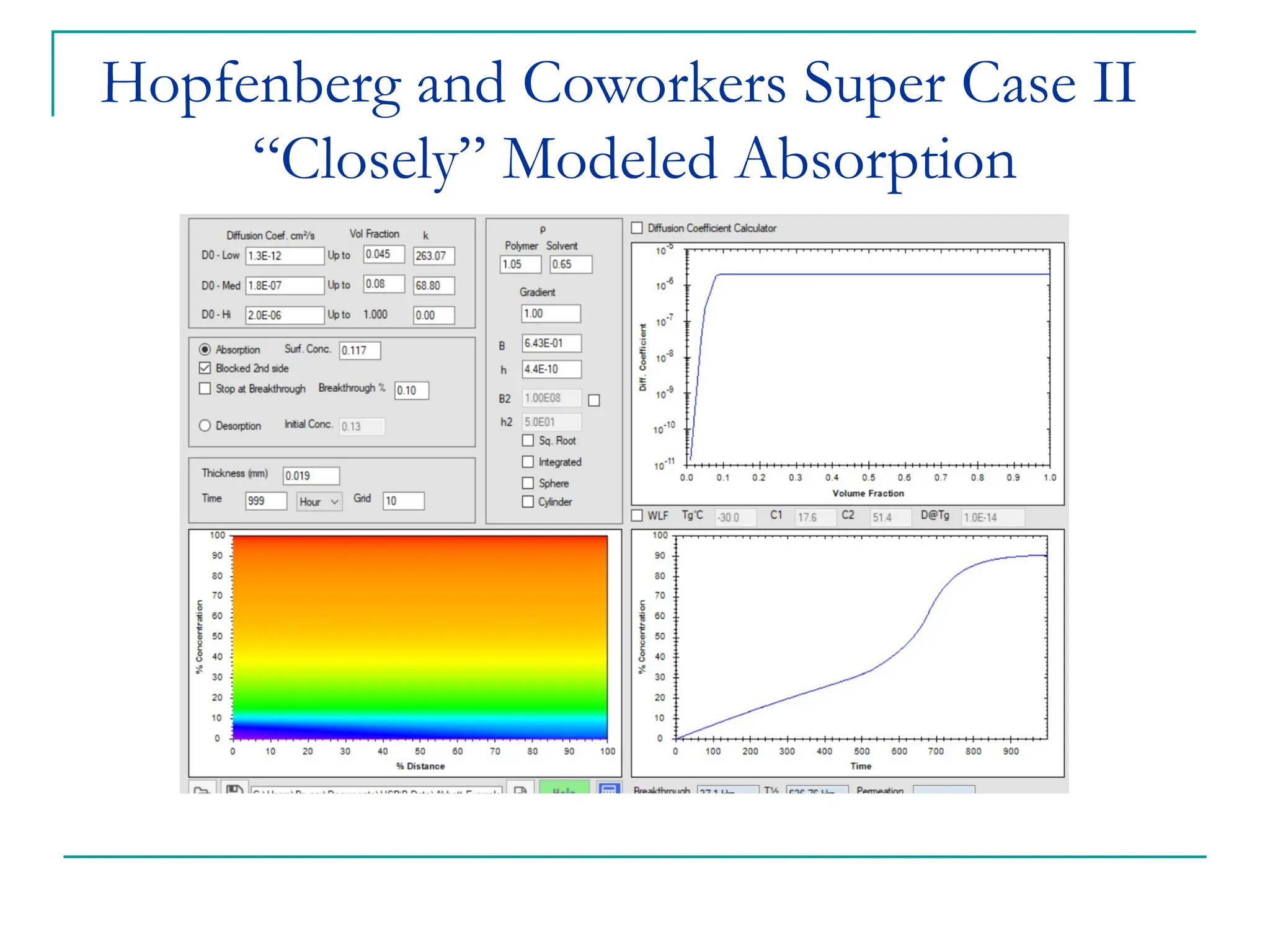
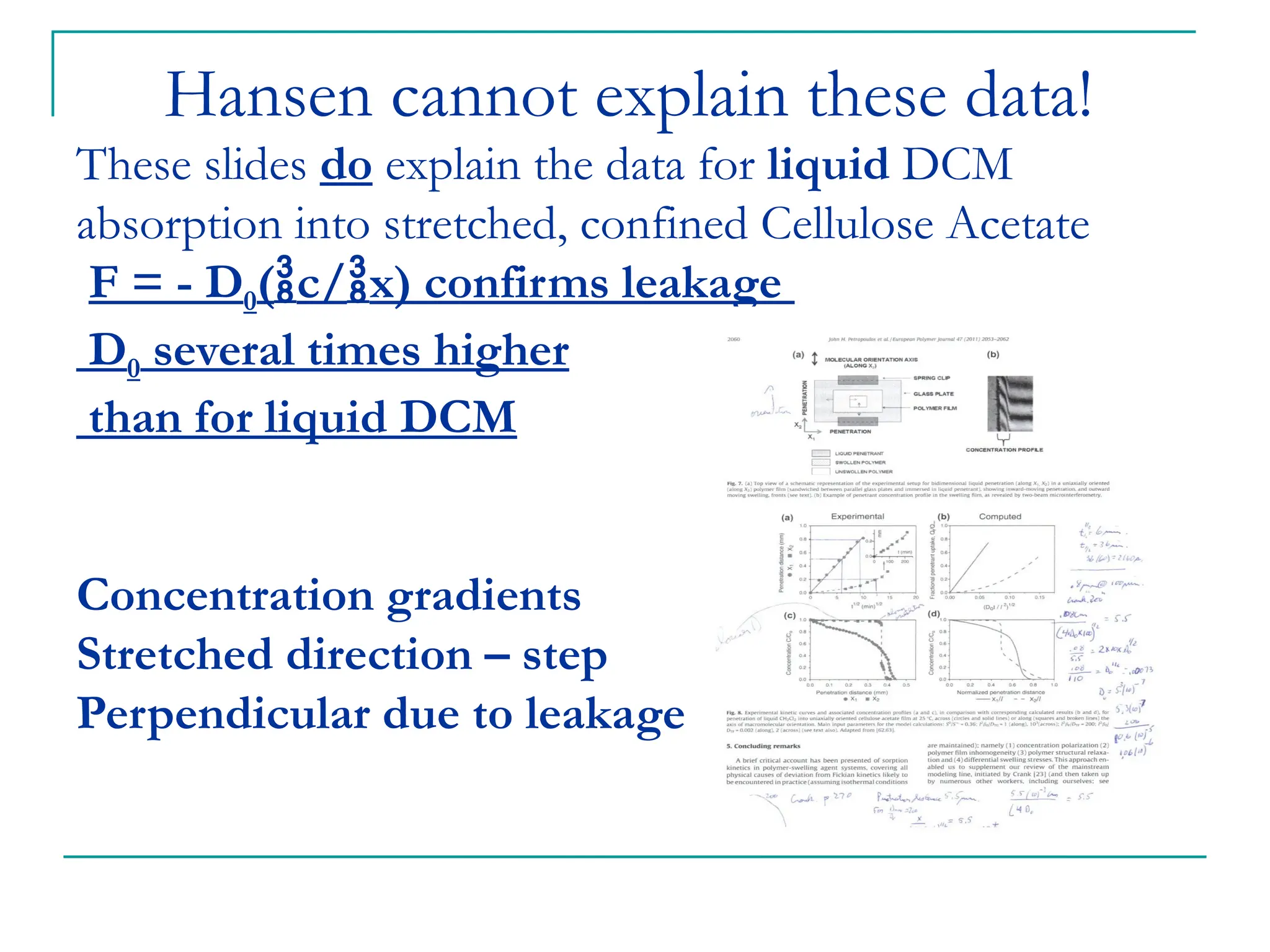
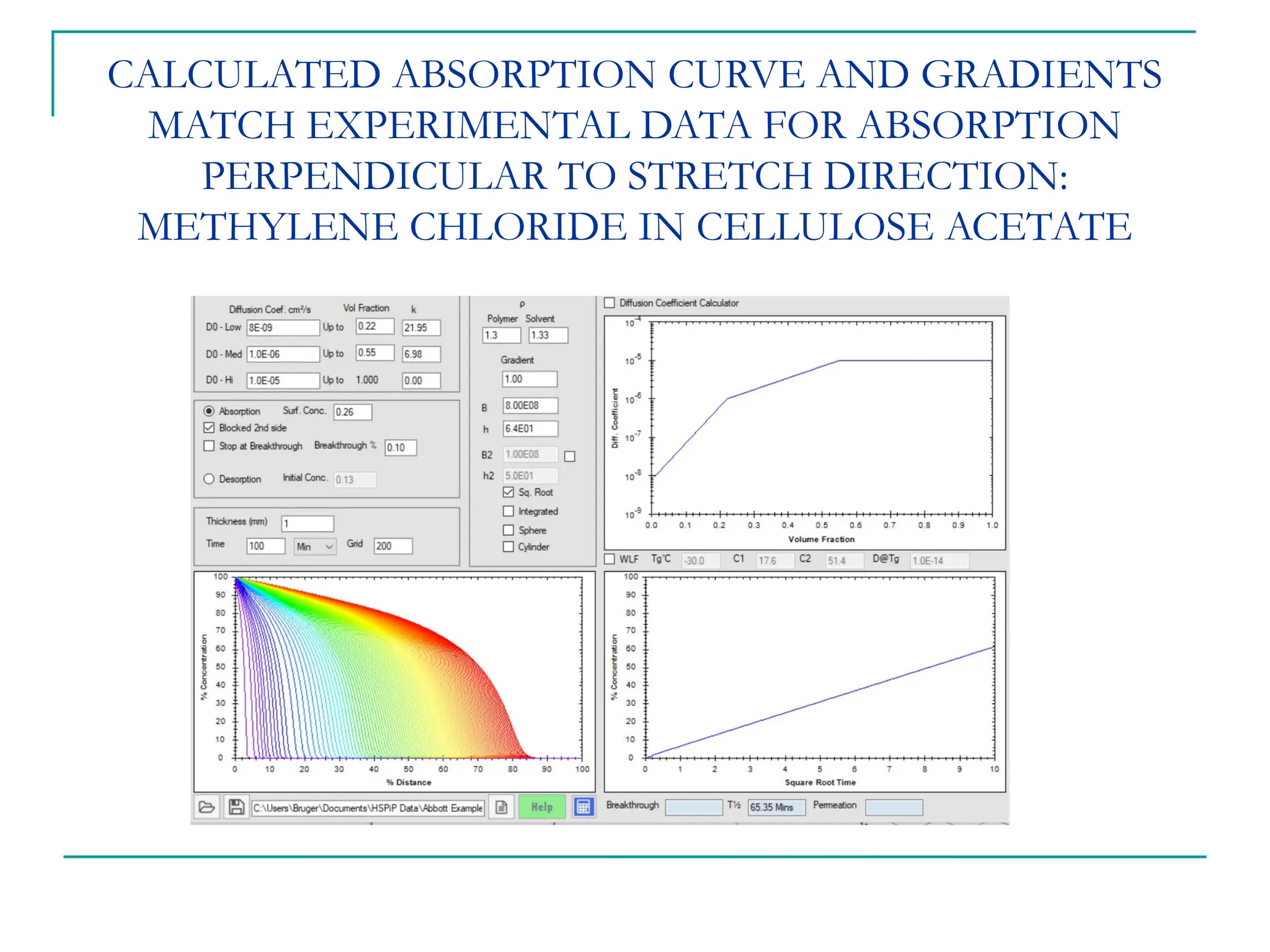
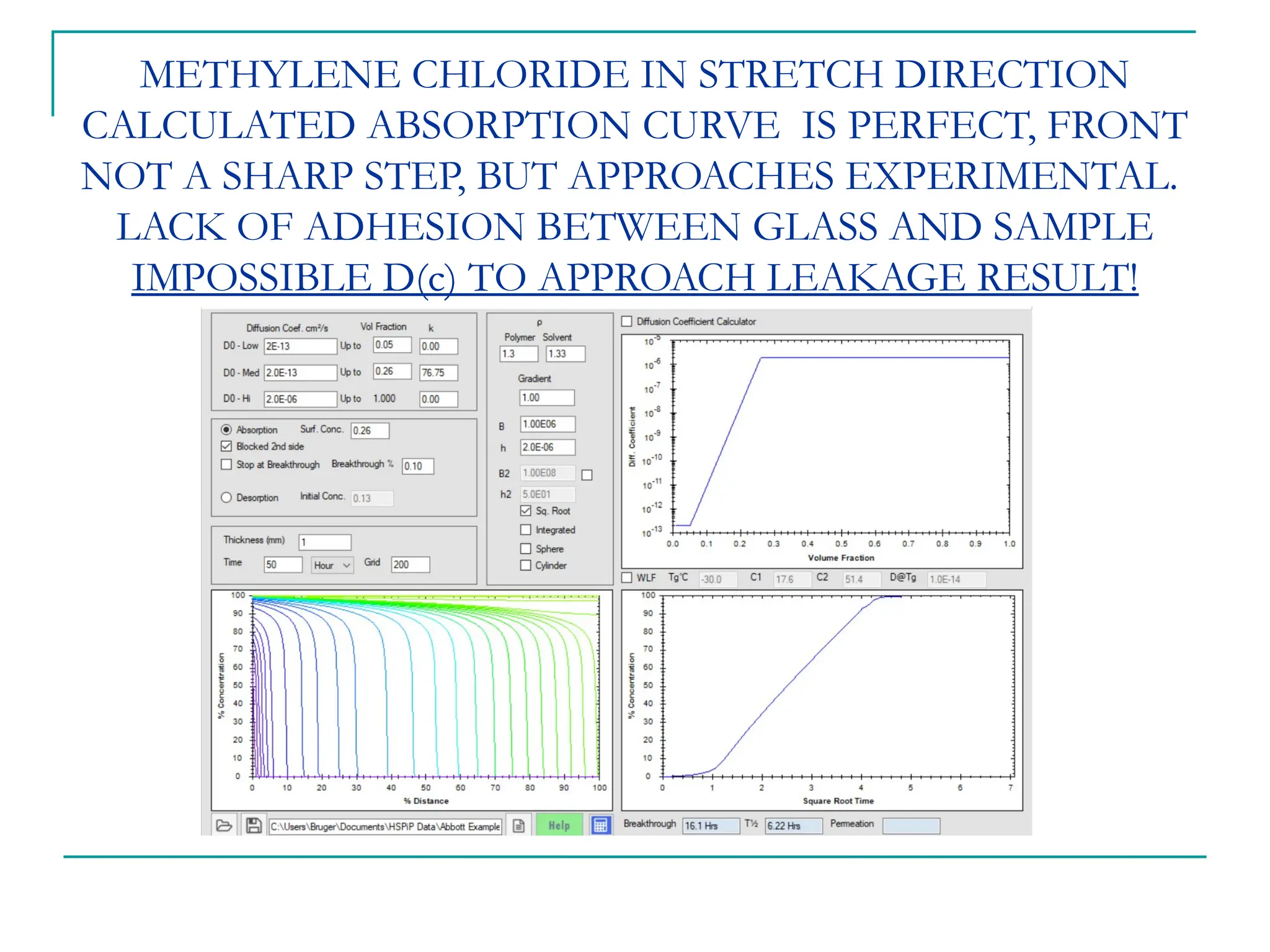
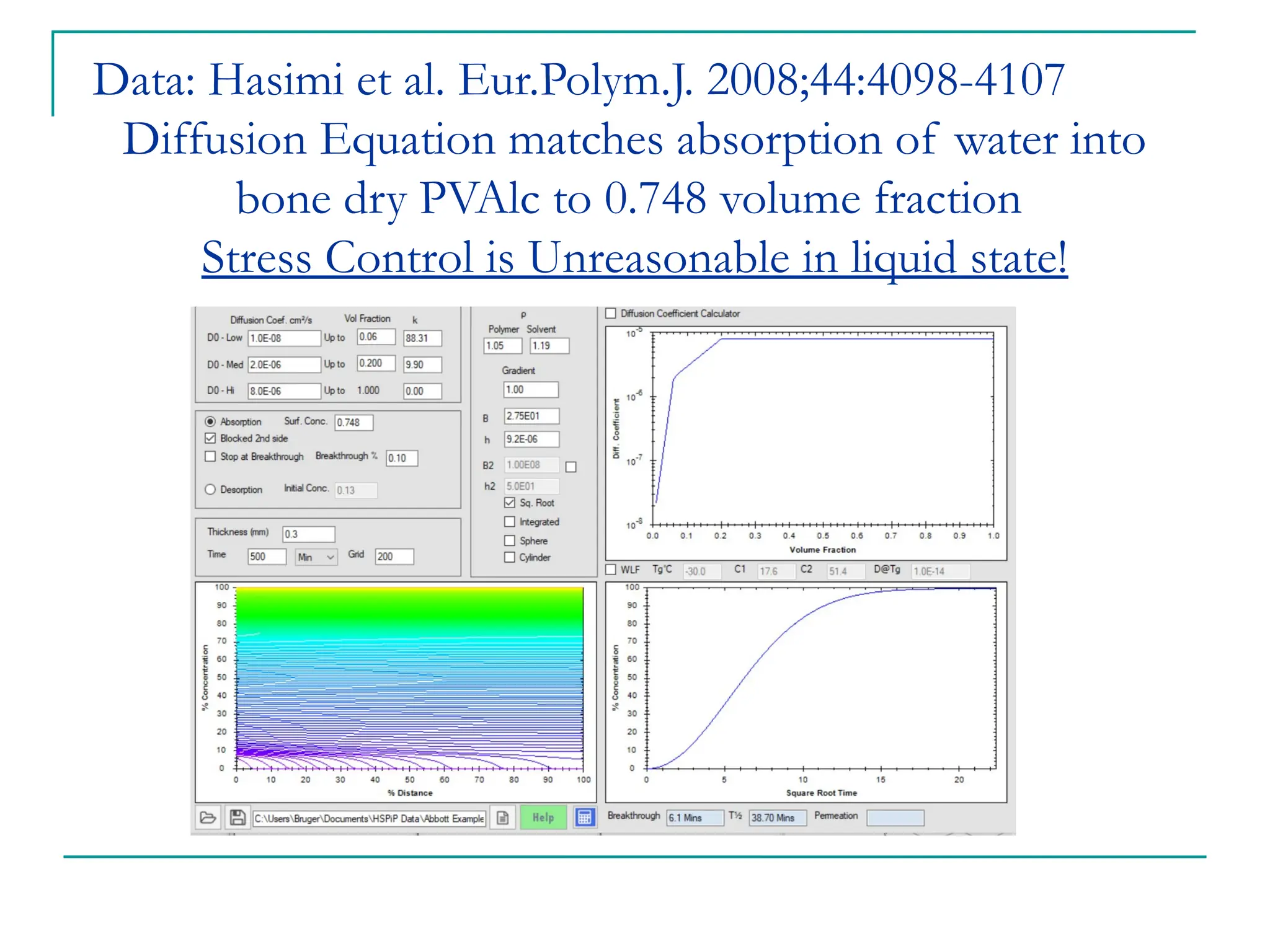
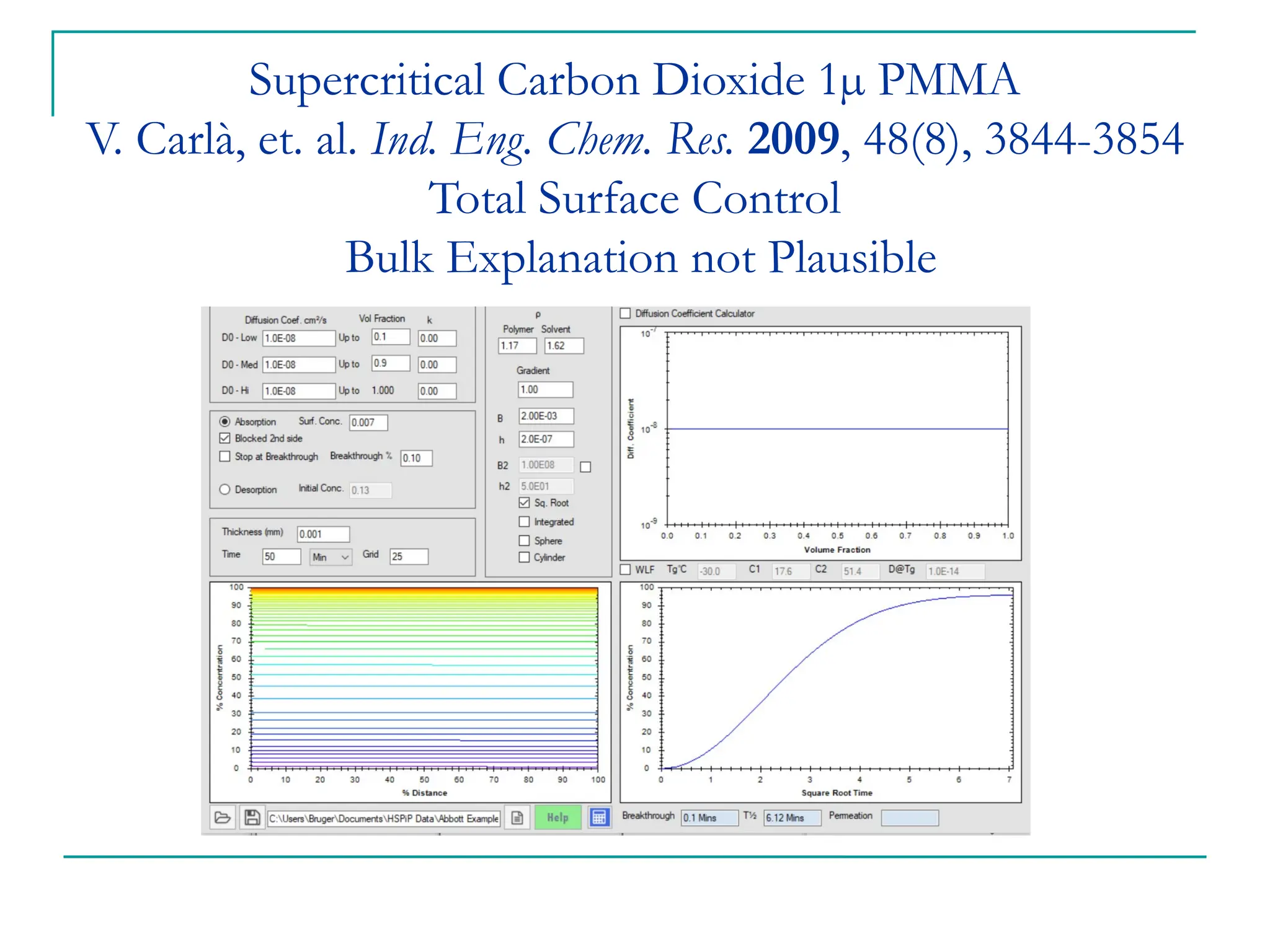
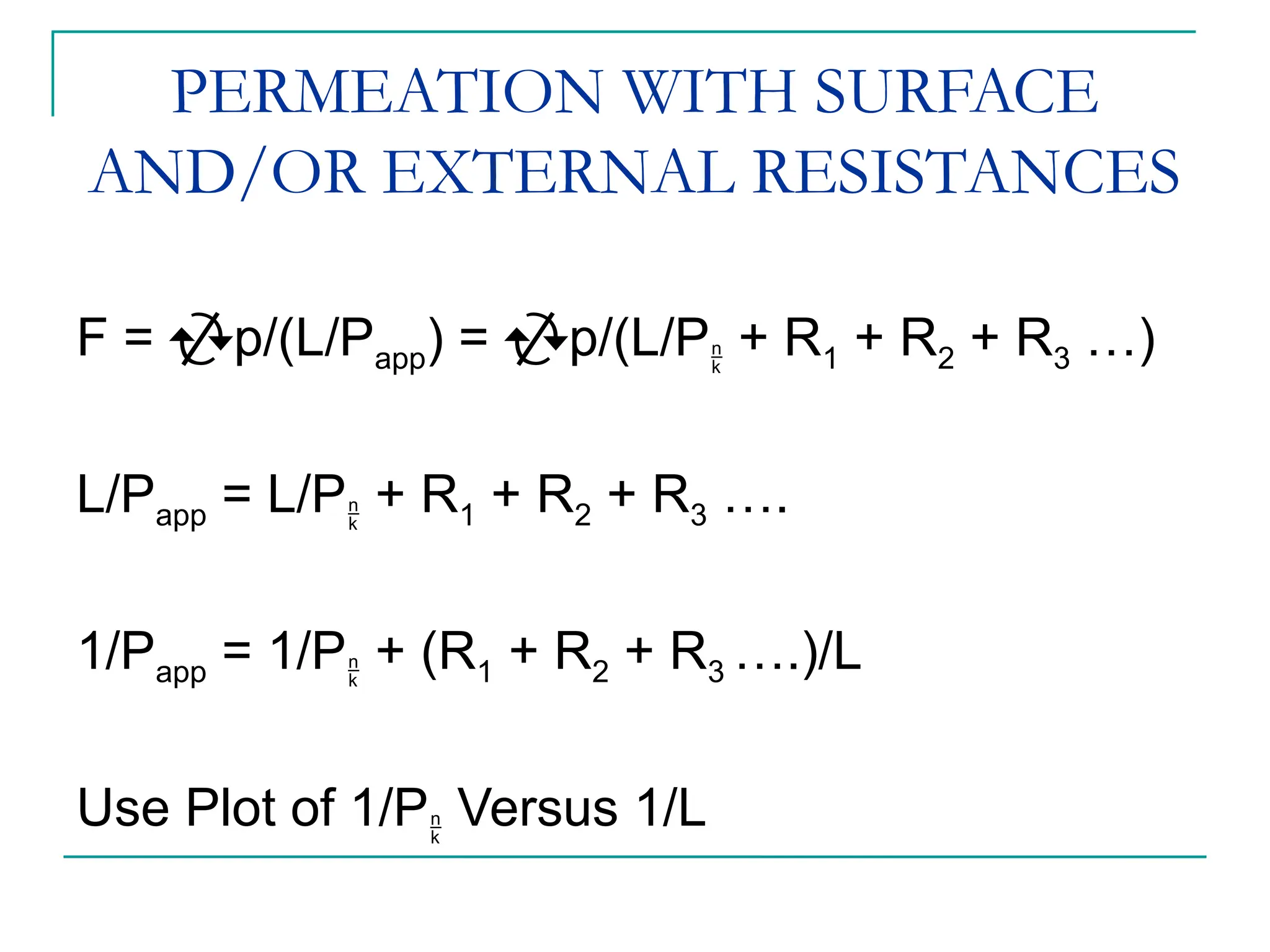
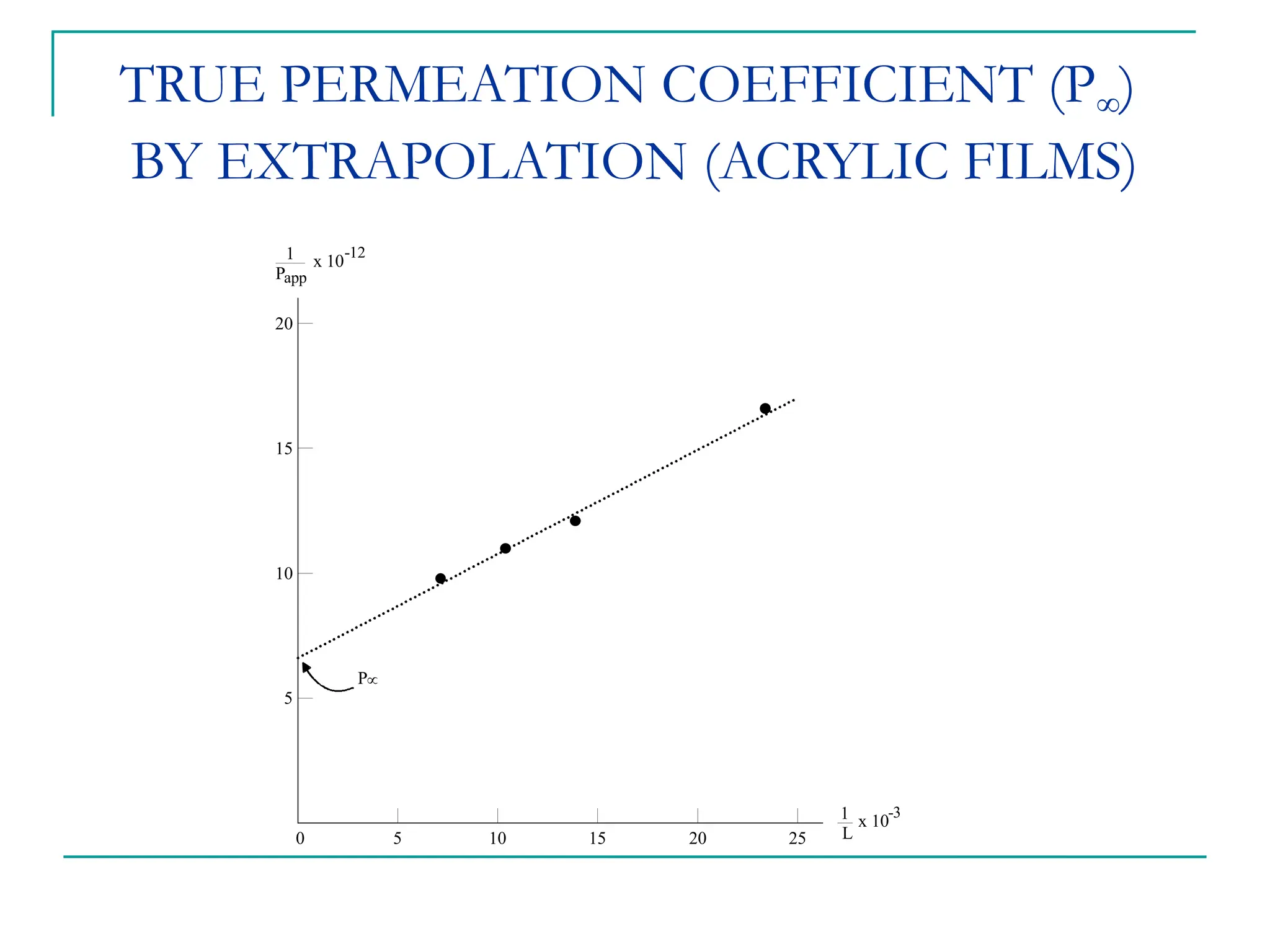
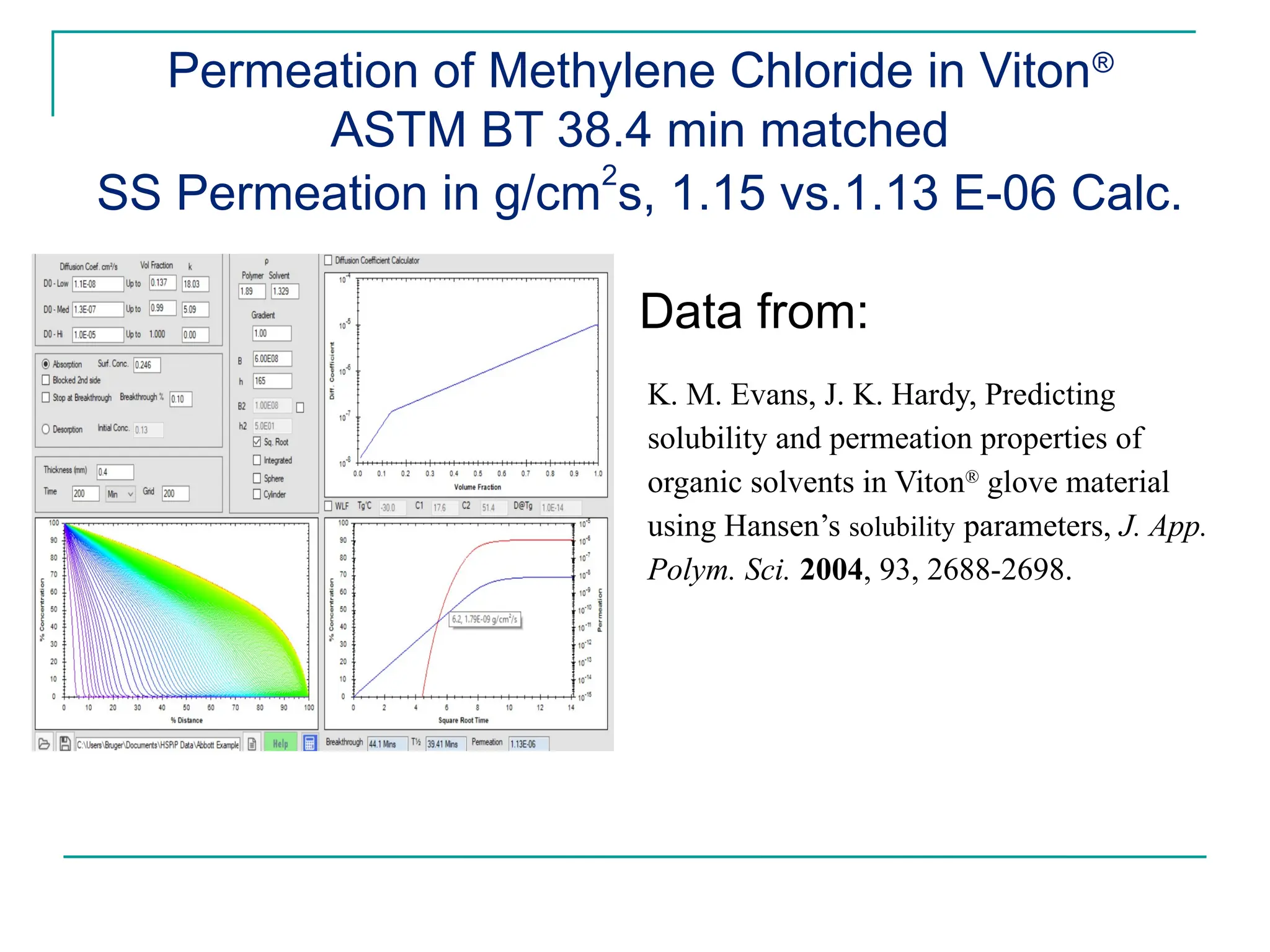
The document discusses the use of the diffusion equation to model various diffusion processes in polymers, focusing on solvent absorption, desorption, and permeation. It emphasizes the importance of concentration-dependent diffusion coefficients and surface effects in accurately predicting absorption behavior. The modeling results align with existing literature, demonstrating that the diffusion equation effectively captures complex diffusion phenomena in polymer systems.