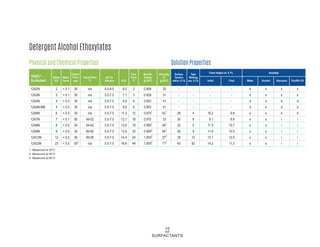
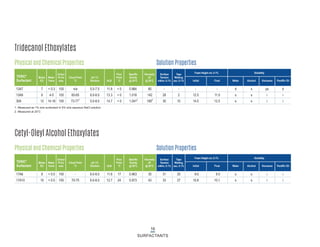
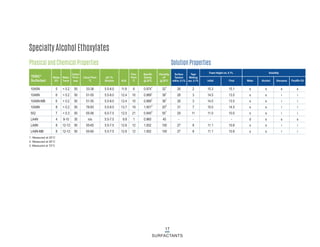
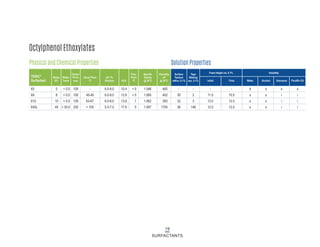
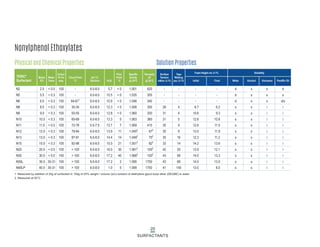
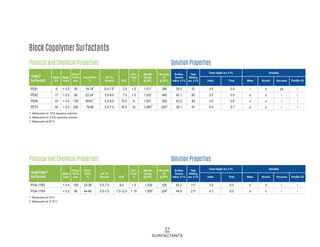
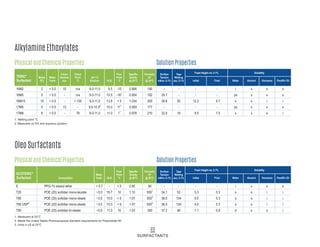
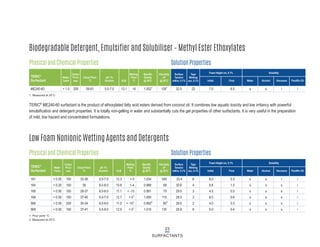
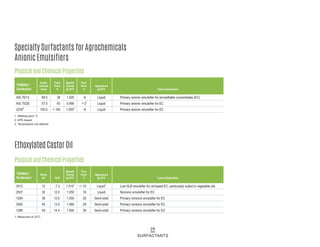
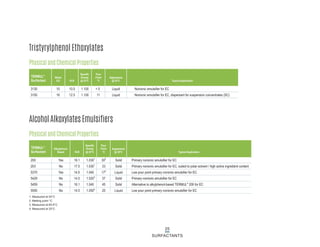
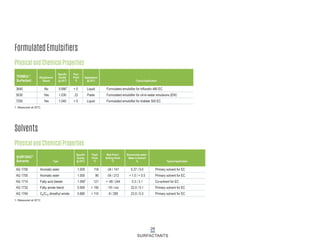
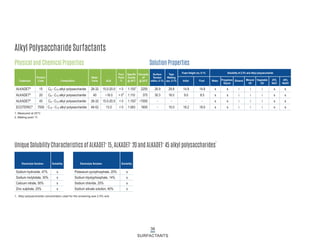
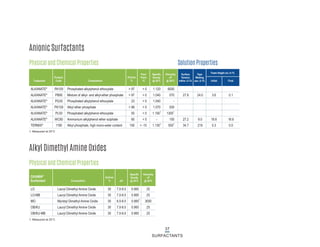
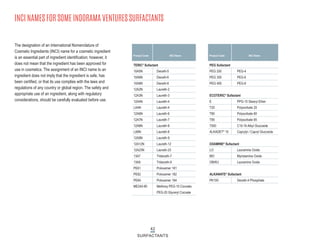
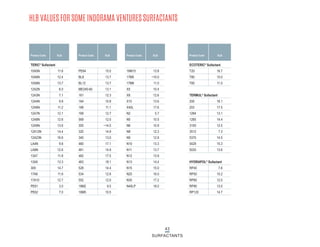
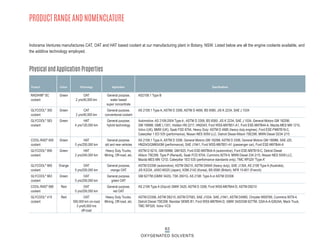
This document provides information on Indorama Ventures' range of nonionic surfactant products. It describes the chemistry of nonionic surfactants, which are produced through the reaction of alkylene oxides like ethylene oxide with alcohols, alkylphenols, or fatty acids. It lists their nonionic surfactants range, including alcohol ethoxylates, alkylphenol ethoxylates, block copolymer surfactants, and oleo surfactants. It also provides product data sheets, applications, INCI names, and handling guidelines for their nonionic surfactant products.