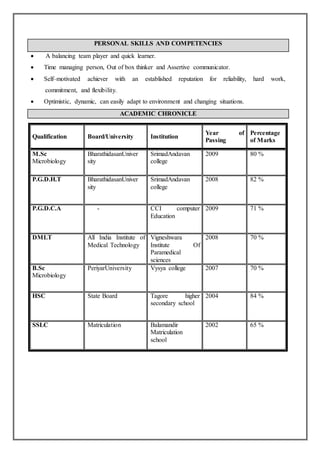
Sivakumar S is applying for a suitable position. He has a Master's degree in Microbiology from Bharathidasan University with distinction. He currently works as a Quality Control Microbiology Team Leader at Reckitt Benckiser in Mysore. He has over 5 years of experience in quality control microbiology roles at various pharmaceutical companies. He is looking to utilize his skills and knowledge to further his career. He has enclosed his curriculum vitae for consideration.