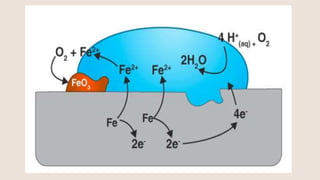
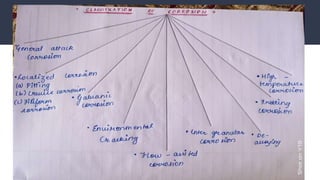
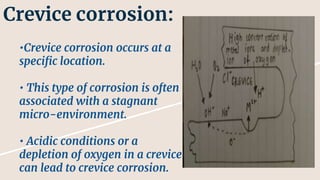
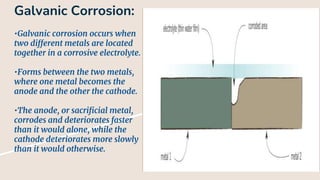
Corrosion is the gradual destruction of materials due to chemical or electrochemical reaction with their environment. Corrosion engineering studies corrosion mechanisms and works to prevent or control corrosion economically and safely. There are two main types of corrosion - general attack corrosion which uniformly destroys metal over time, and localized corrosion which targets specific areas. Localized corrosion includes pitting, crevice corrosion, filiform corrosion, galvanic corrosion, and others. Proper material selection and protective coatings can help prevent corrosion in pharmaceutical facilities and equipment.