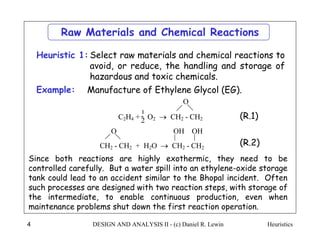
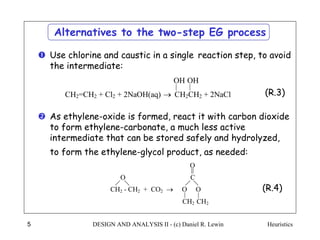
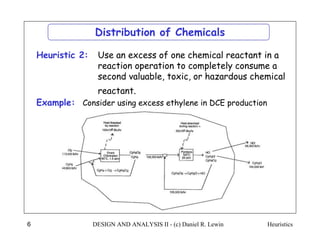
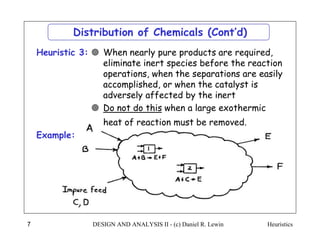
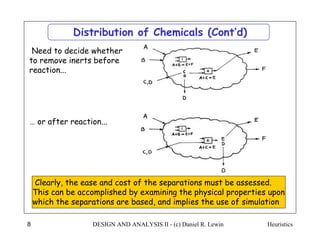
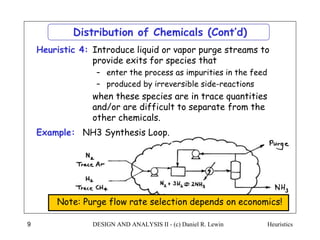
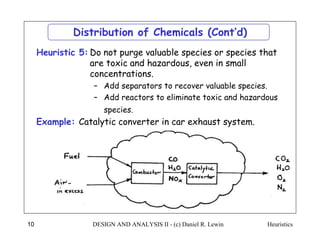
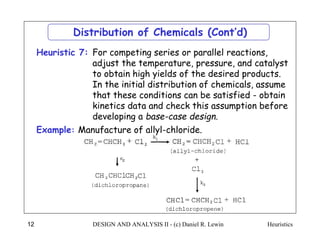
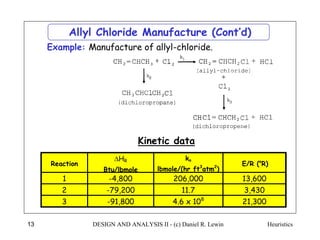
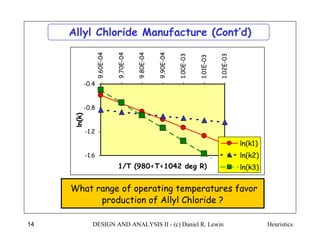
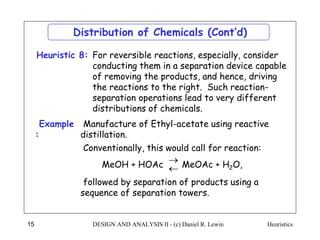
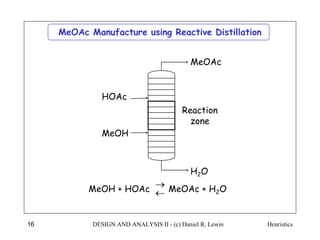
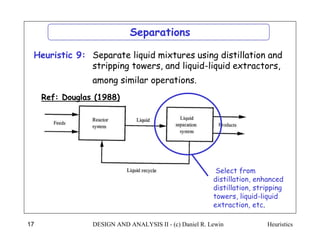
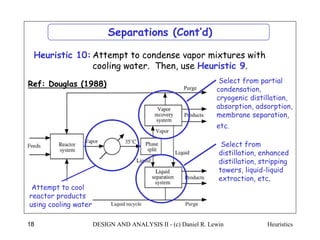
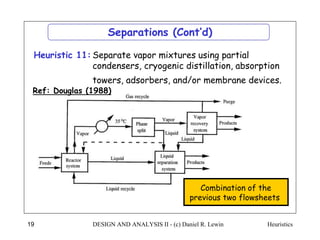
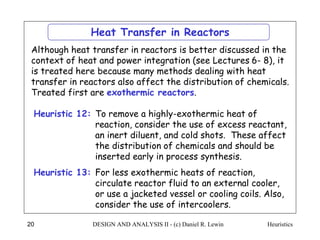
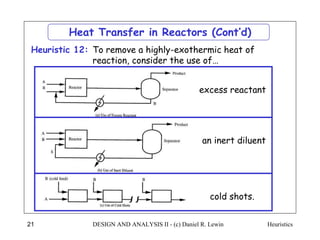
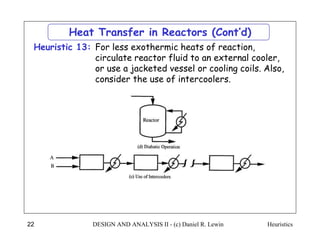
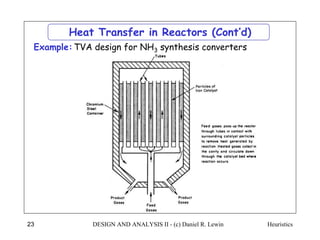
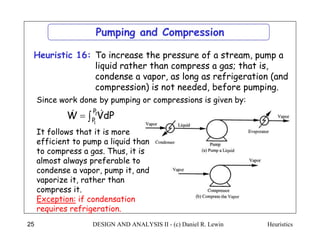
The document discusses various heuristics for process synthesis and distribution of chemicals in process flowsheets. Some key heuristics covered include selecting reactions and raw materials to avoid hazardous chemicals, using excess reactants to consume toxic reactants, introducing purge streams for impurities, adjusting temperature and catalysts to favor desired reactions, and using techniques like excess reactants, inert diluents, and cold shots to handle highly exothermic heat of reactions. The heuristics provide general guidelines for assembling processing operations and distributing chemicals in a flowsheet.