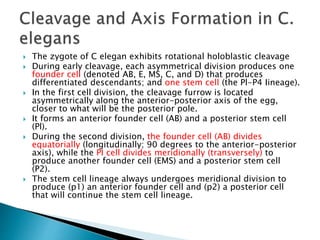
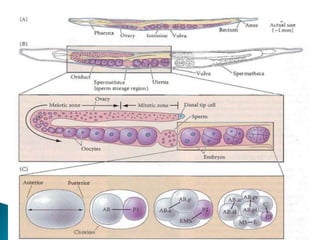
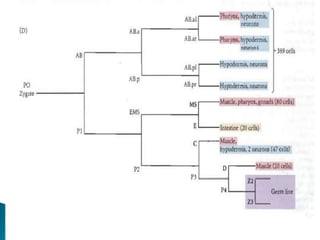
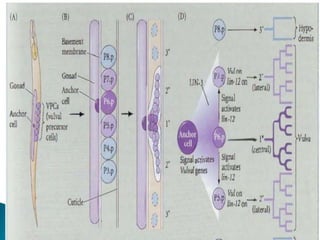
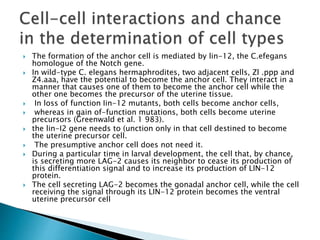
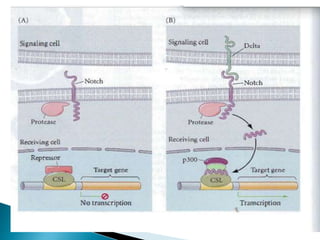
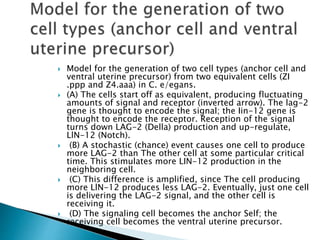
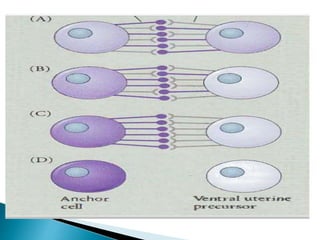
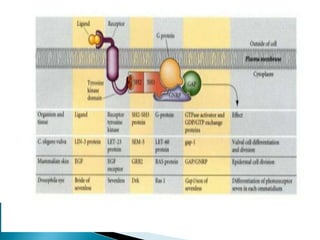
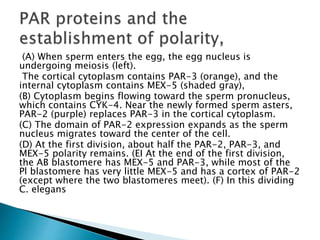
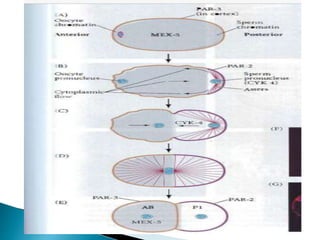
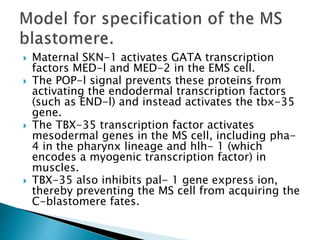
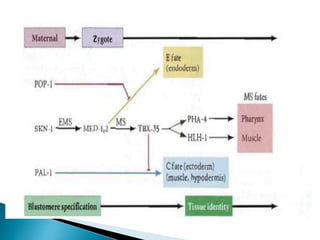
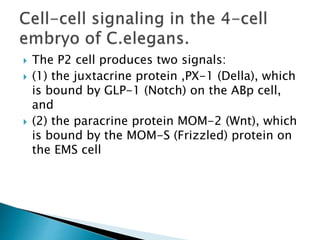
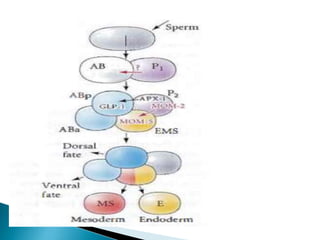
The document summarizes cell division and differentiation in the early C. elegans embryo. It describes how asymmetrical cell divisions produce founder cells that give rise to distinct lineages, and how signaling between cells determines their fates. Specifically, it discusses how PAR and MEX proteins establish polarity in the zygote, LIN-3/LET-23 signaling specifies vulval precursor cell fates, and LAG-2/LIN-12 and PX-1/GLP-1 interactions determine cell identities in later embryonic lineages.