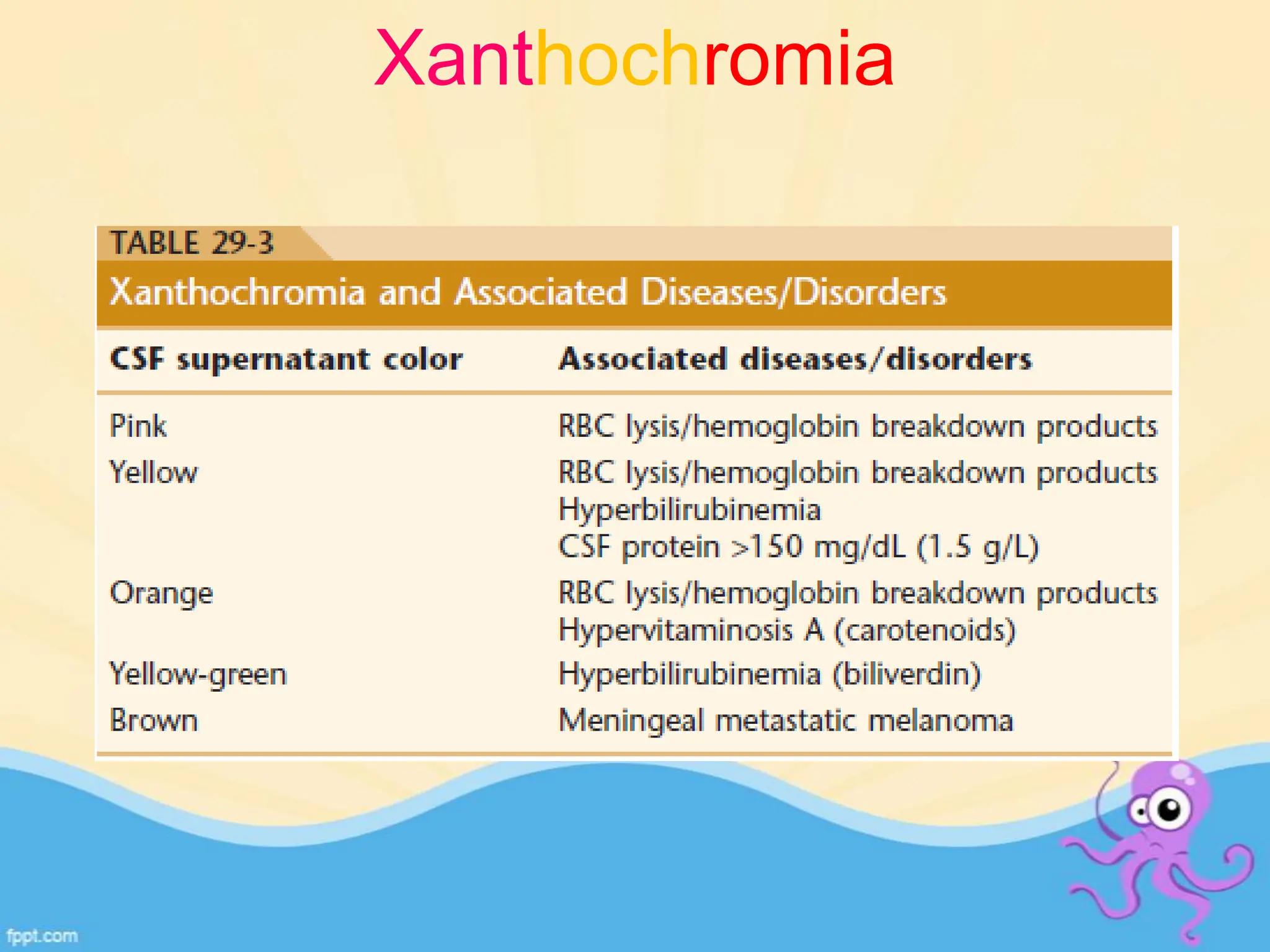
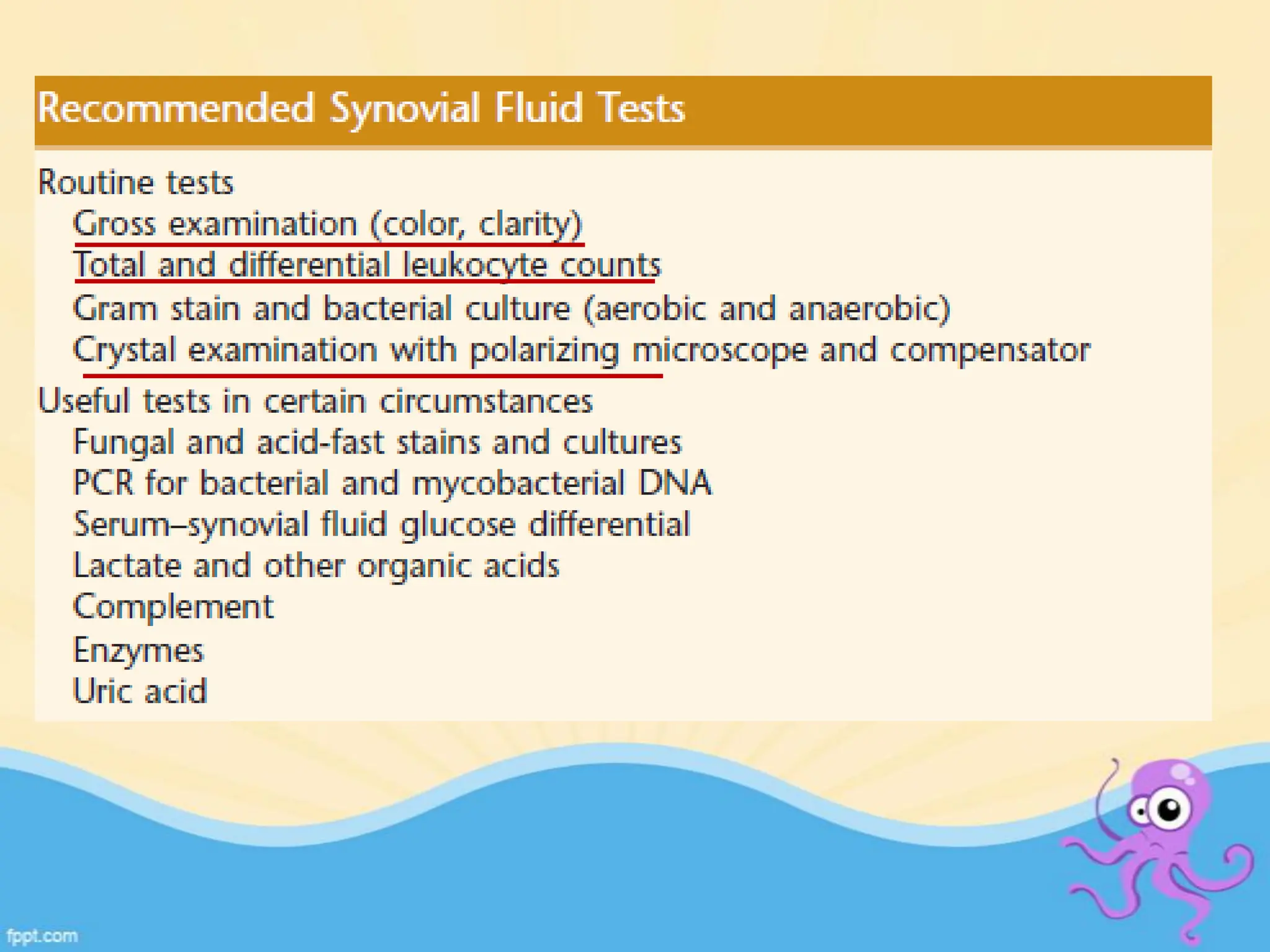
The document provides an overview of body fluid analysis in clinical laboratories, detailing collection methods, turnaround times, communication between physicians and labs, and reference values for various fluids including cerebrospinal, synovial, and serous fluids. It outlines testing reasons, specimen collection procedures, microscopic examination techniques, and ancillary techniques such as flow cytometry. Additionally, it emphasizes the importance of reliable analysis to detect conditions like meningitis, malignancies, and systemic diseases.