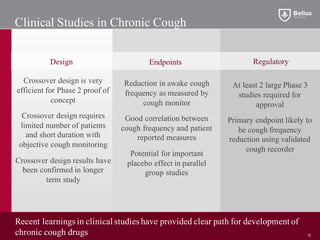
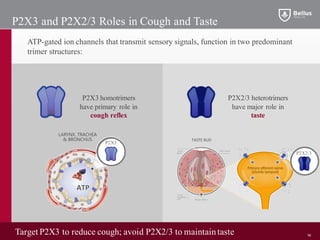
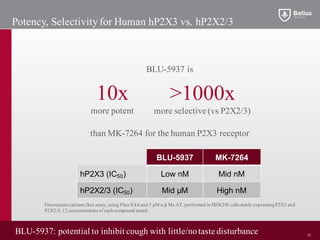
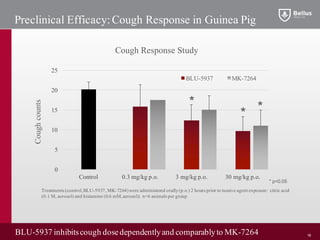
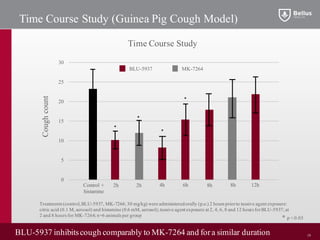
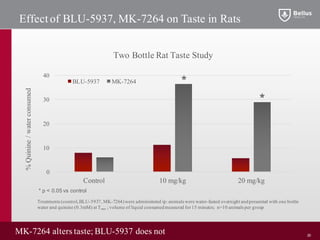
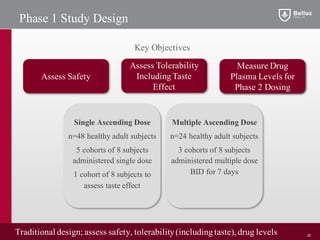
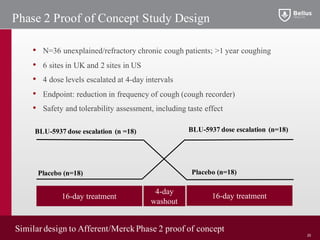
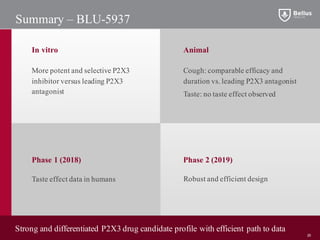
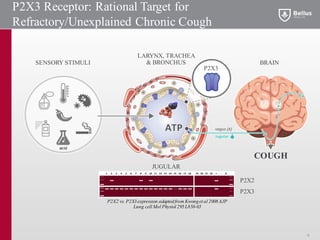
BLU-5937 is a P2X3 receptor antagonist being developed by BELLUS Health for the treatment of chronic cough. P2X3 receptors are involved in cough signaling and are a validated target for chronic cough therapies. In preclinical studies, BLU-5937 showed potent and selective inhibition of the P2X3 receptor with no effect on taste, unlike leading P2X3 antagonist MK-7264 which caused taste disturbances. BLU-5937 inhibited cough in guinea pig models comparably to MK-7264 but with a wider safety margin. BELLUS Health is conducting a Phase 1 trial in 2018 to assess the safety, tolerability and pharmacokinetics of BLU-59




![P2X3 Receptor: Clinically Validated Target
Targeting P2X3 is an efficacious strategyfor treatingchronic cough
Merck & Co., Inc. (2017). Merck Announces Presentation of Phase 2 Results for MK-7264, an Investigational, P2X3 Receptor Antagonist, Being Evaluated for the Treatment of Chronic Cough. [Press Release]. Retrieved from
http://www.mrknewsroom.com/news-release/research-and-development-news/merck-announces-presentation-phase-2-results-mk-7264-inve
Merck’s MK-7264 - P2X3 Antagonist
Reduction in Awake Cough Frequency
(from Baseline Compared to Placebo)
* p<0.05 vs. placebo
0%
20%
40%
60%
80%
Placebo 7.5 mg 20 mg 50 mg
*
Phase IIb (253 patients; 12
week study) showed
reduction in awake cough
frequency of
84% vs baseline
37% vs placebo
at 50mg dose
10](https://image.slidesharecdn.com/blu-5937koleventsept20vfinal-170920170911/85/Blu-5937-kol-event-sept-20-v-final-10-320.jpg)
![MK-7264: Significant Adverse Taste Effect
Taste effect likelydue to low selectivityfor P2X3; MK-7264 also inhibiting
P2X2/3, particularlyat 50mg dose
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
Placebo 7.5mg 20mg 50mg
Taste Disturbance Complete Loss of Taste
At therapeutic dose (50 mg BID):
~80%
of patients
reported taste
alteration
~40%
of patients reported
very/extremely
bothersome taste
effect
Merck & Co., Inc. (2017). Merck Announces Presentation of Phase 2 Results for MK-7264, an Investigational, P2X3 Receptor Antagonist, Being Evaluated for the Treatment of Chronic Cough. [Press Release]. Retrieved from
http://www.mrknewsroom.com/news-release/research-and-development-news/merck-announces-presentation-phase-2-results-mk-7264-inve
Phase IIb: Percent of Patients Reporting Taste
Side Effect
11](https://image.slidesharecdn.com/blu-5937koleventsept20vfinal-170920170911/85/Blu-5937-kol-event-sept-20-v-final-11-320.jpg)