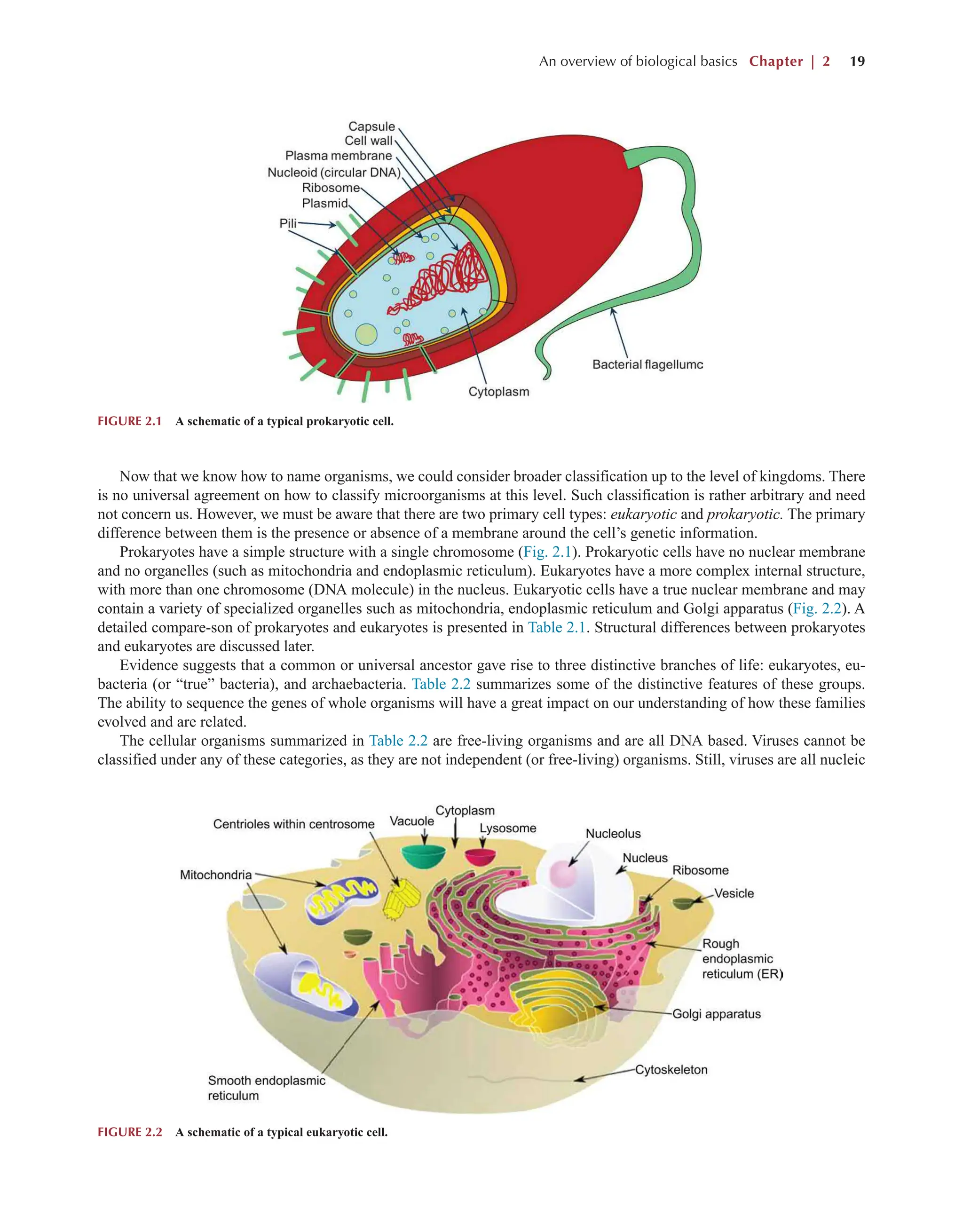
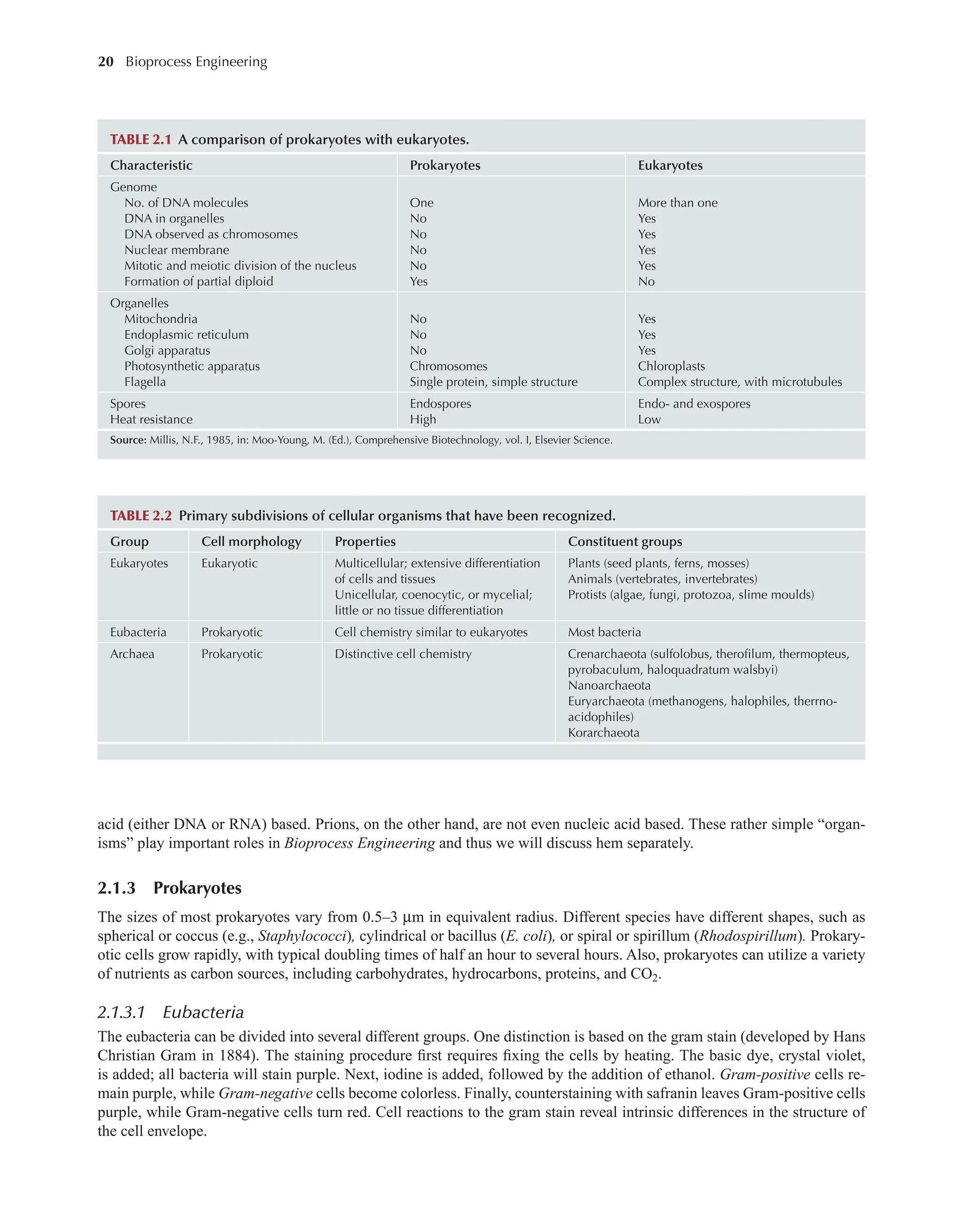
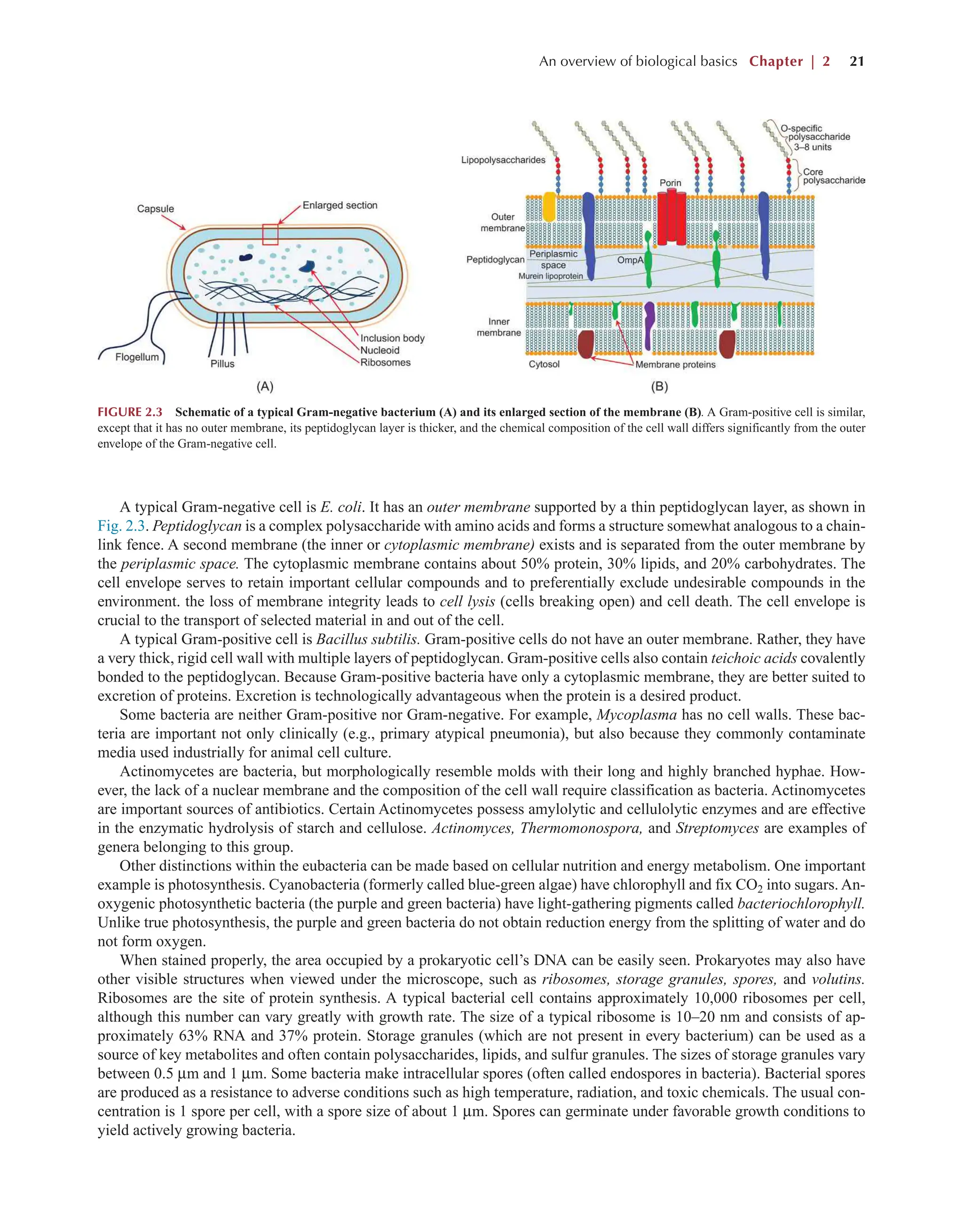
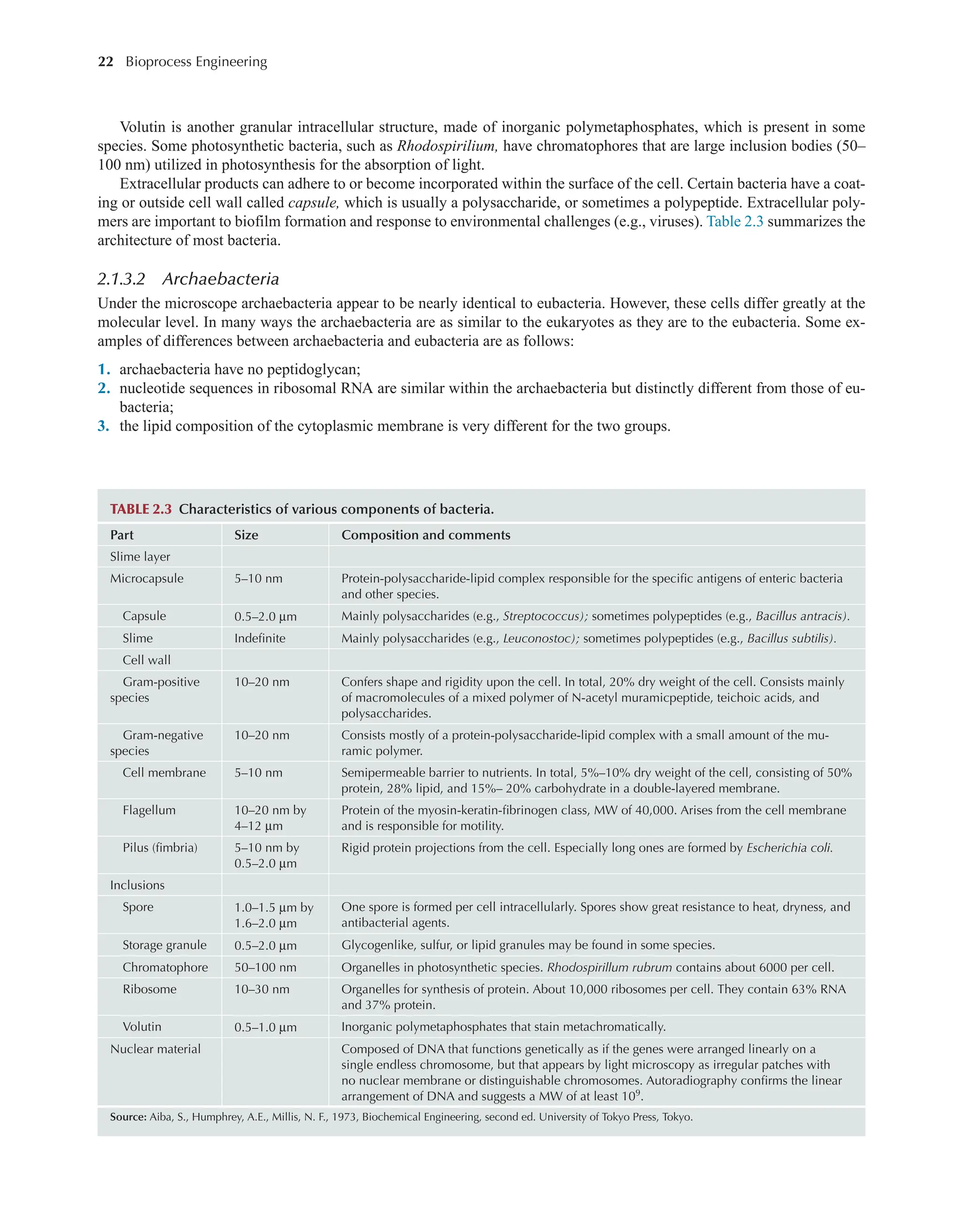
Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design 3rd Edition Shijie Liu
Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design 3rd Edition Shijie Liu
Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design 3rd Edition Shijie Liu