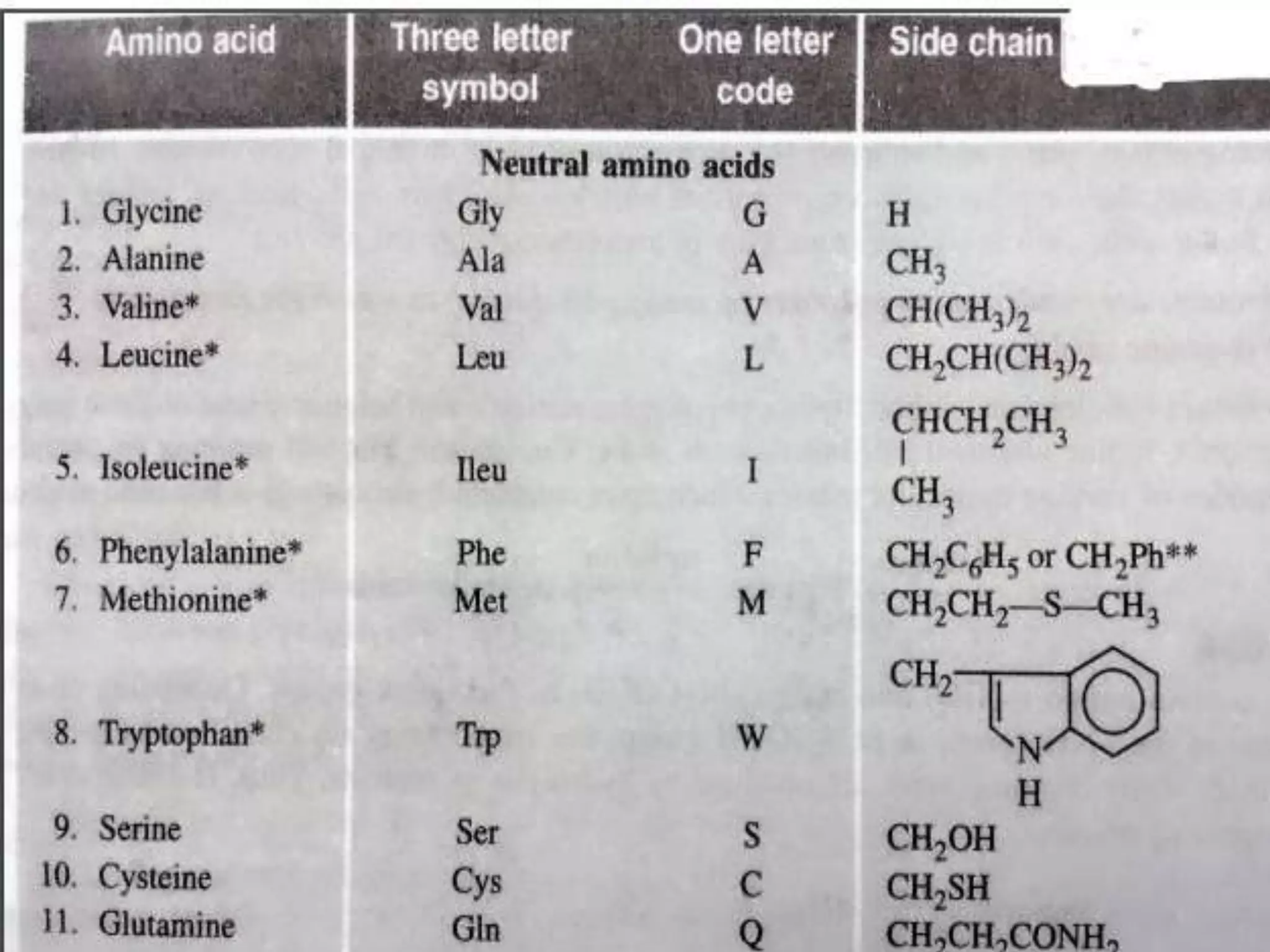
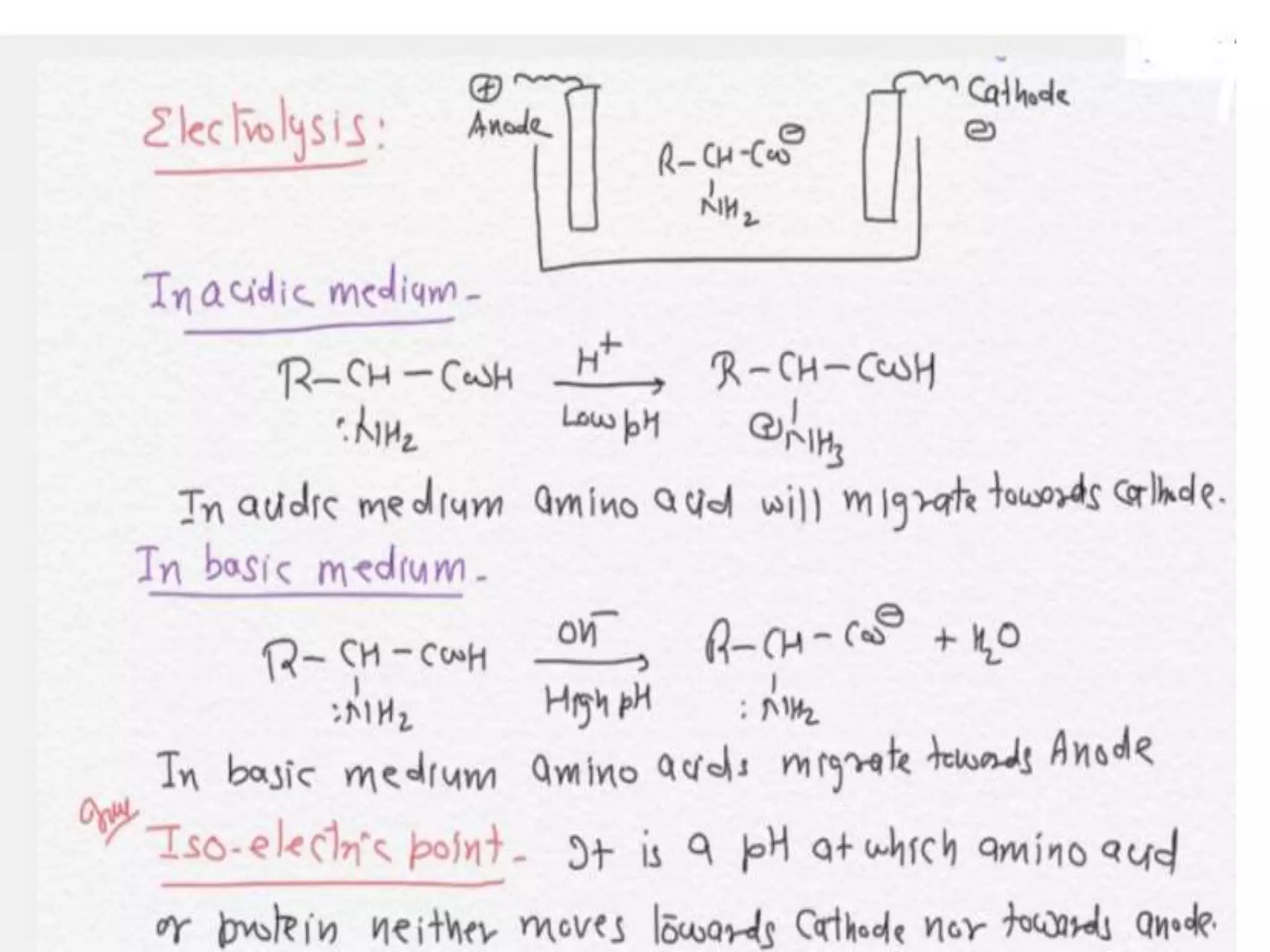
The document discusses different types of biomolecules including carbohydrates, proteins, and nucleic acids. Carbohydrates are classified based on their monomer units into monosaccharides, oligosaccharides, and polysaccharides. They are also classified based on their reducing properties and functional groups. Proteins are made of amino acids and can form various structures like alpha helices due to hydrogen bonding. Nucleic acids like DNA and RNA are made of nucleotides and carry genetic information.