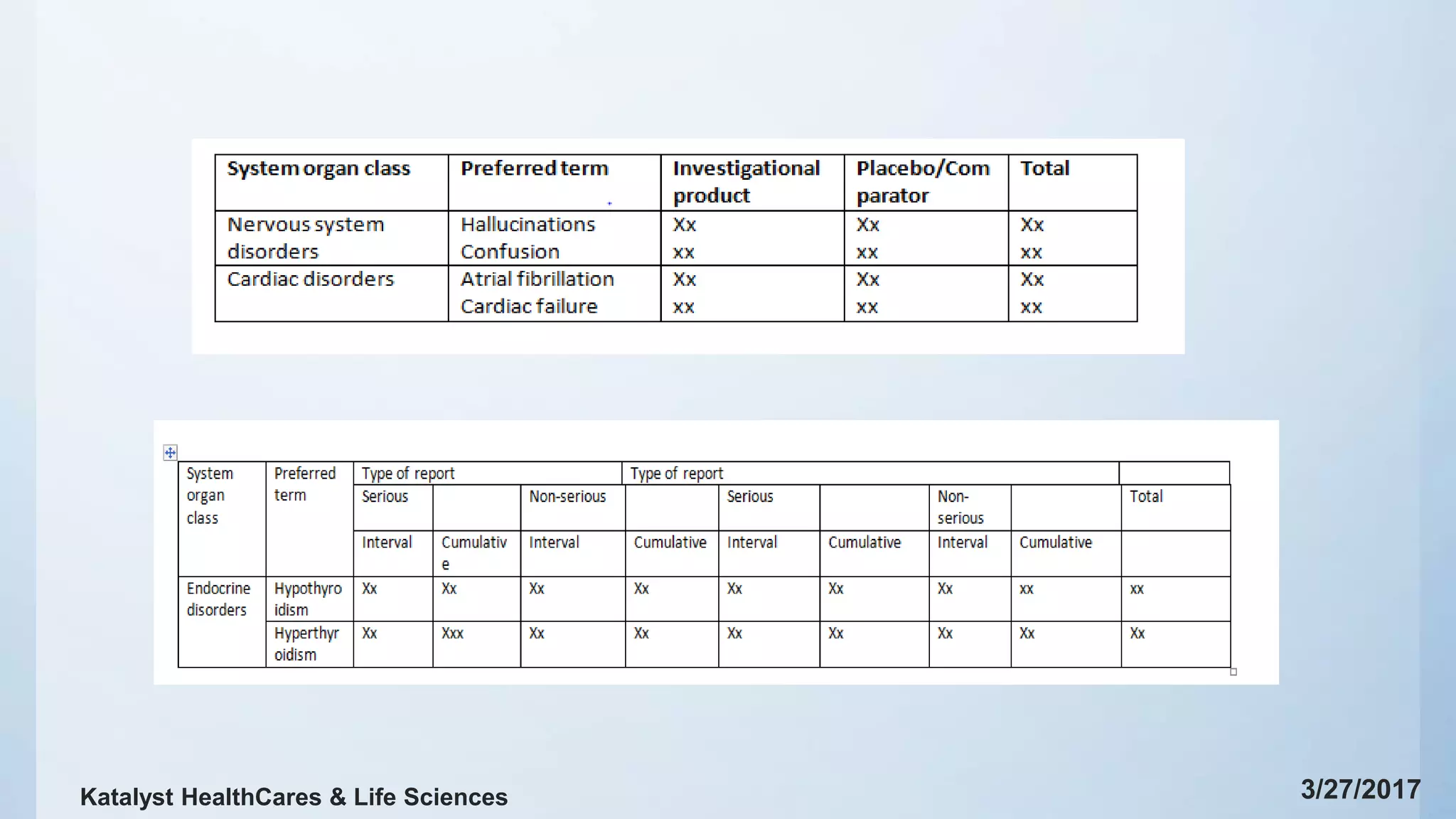
The document provides a comprehensive overview of aggregate reporting in pharmacovigilance, detailing types of safety reports, submission timelines, and essential terminology. It addresses the objectives of safety evaluations, including benefit-risk analyses and outlines necessary actions taken for safety during reporting intervals. Key sections include worldwide marketing authorization status, data tabulations, and summaries of significant safety findings from clinical trials.