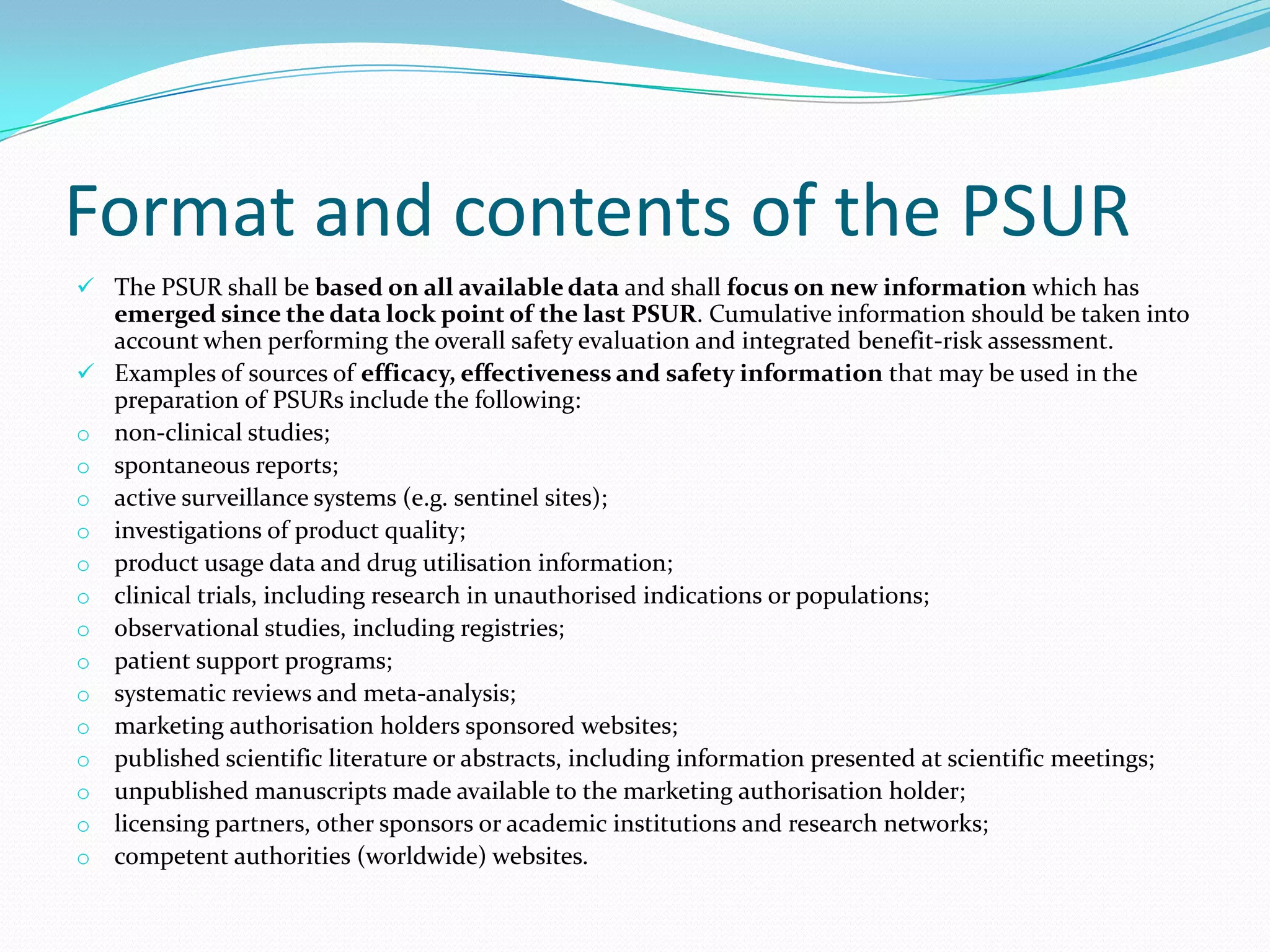
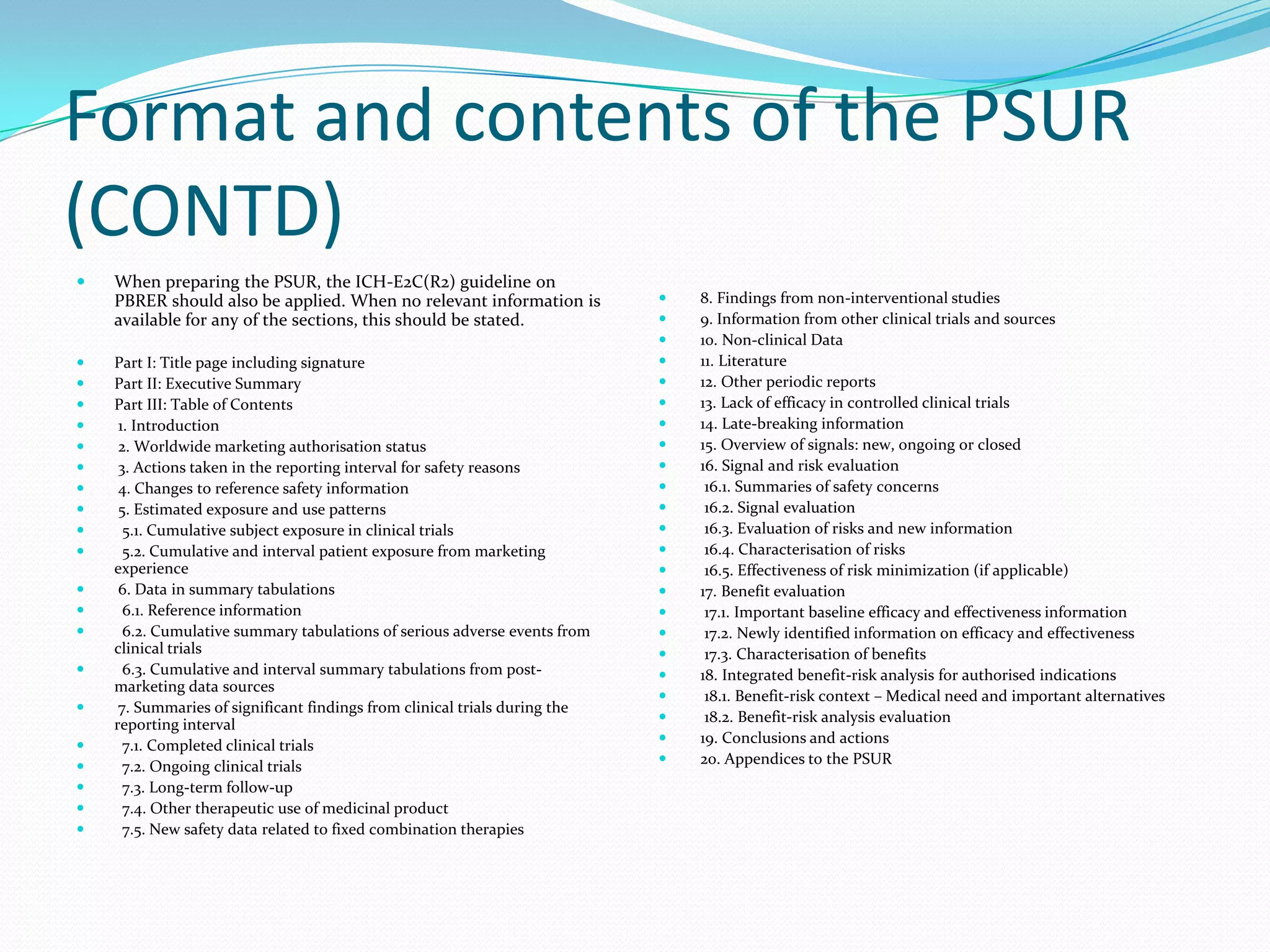
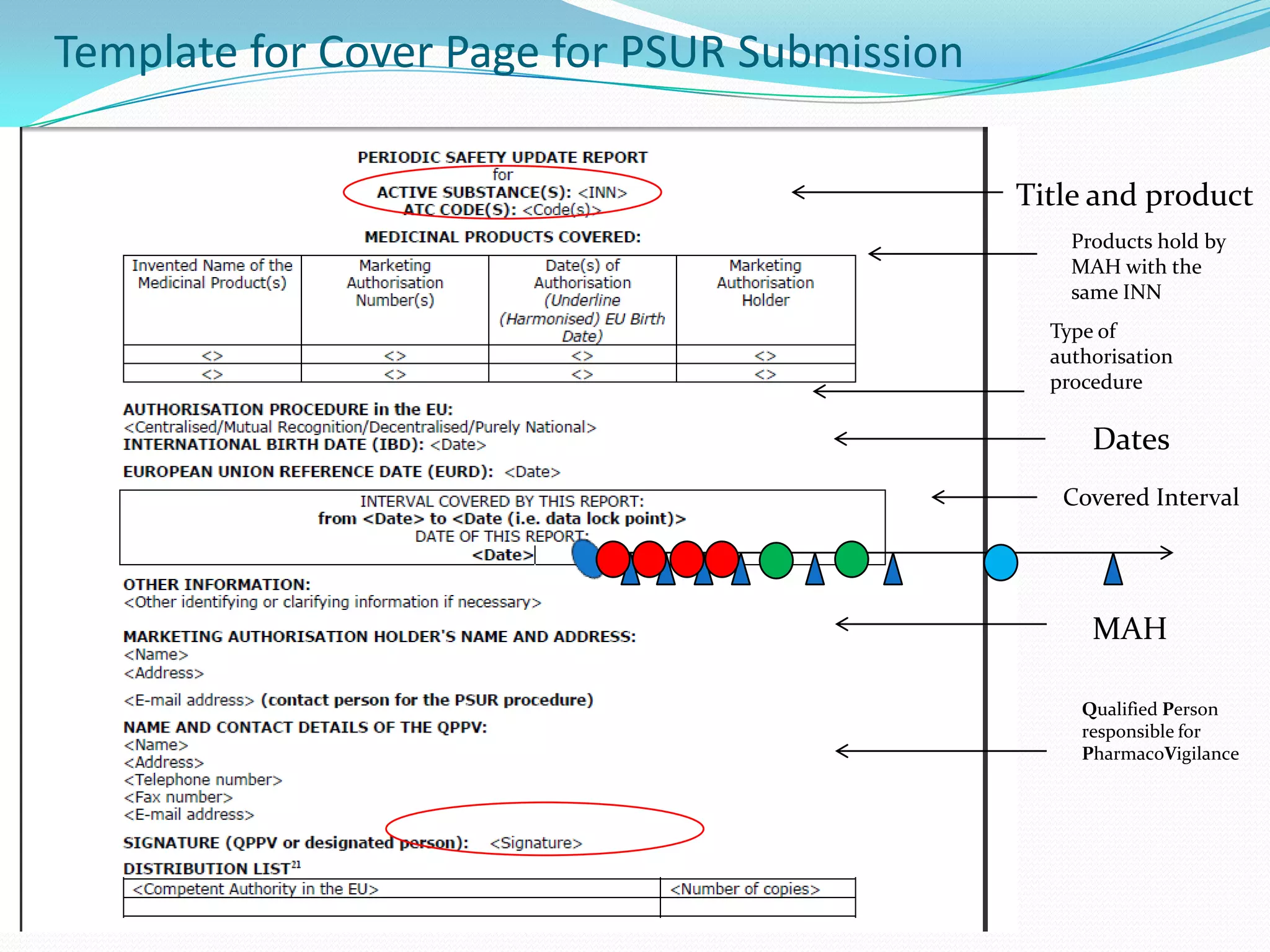
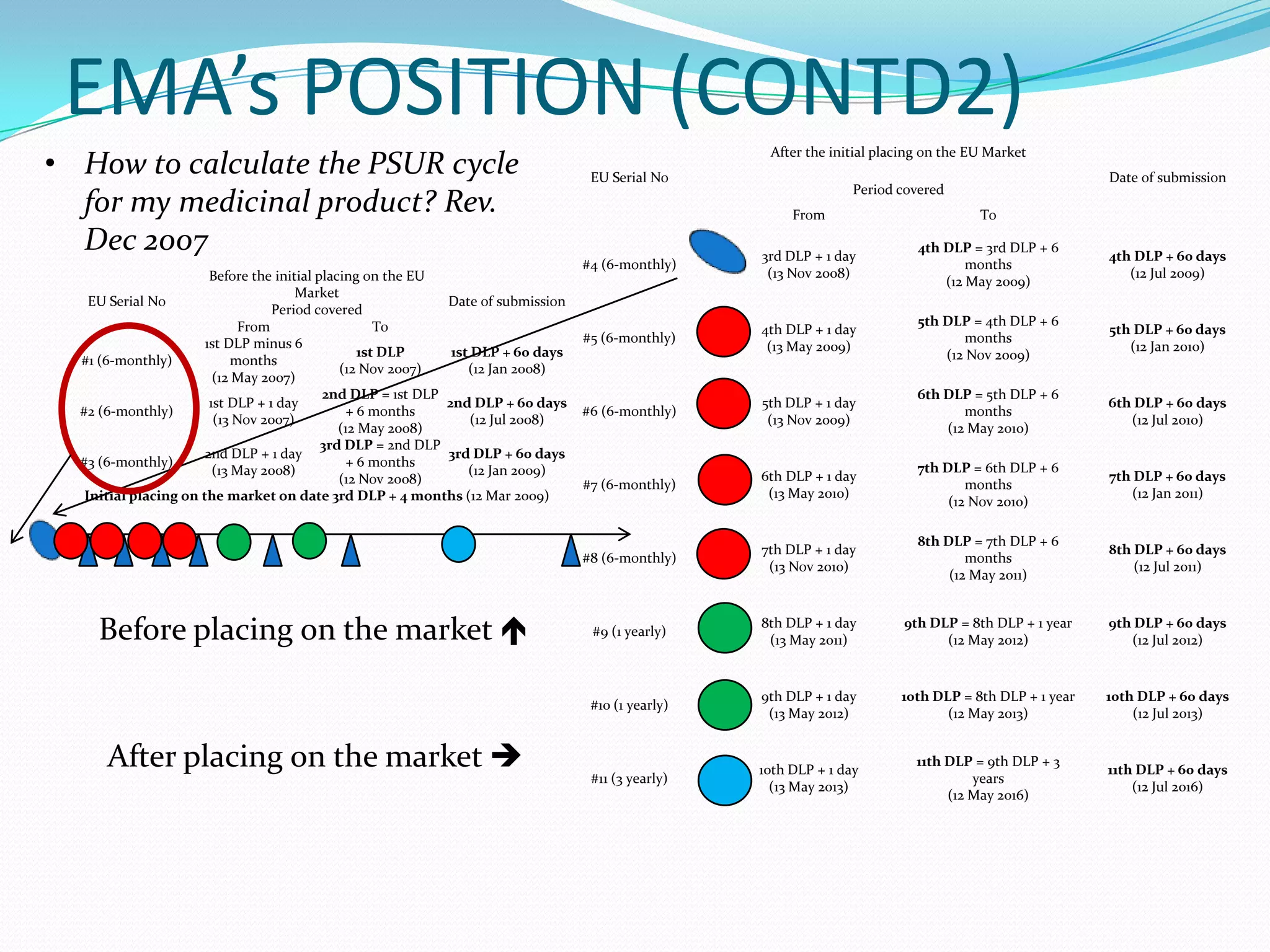
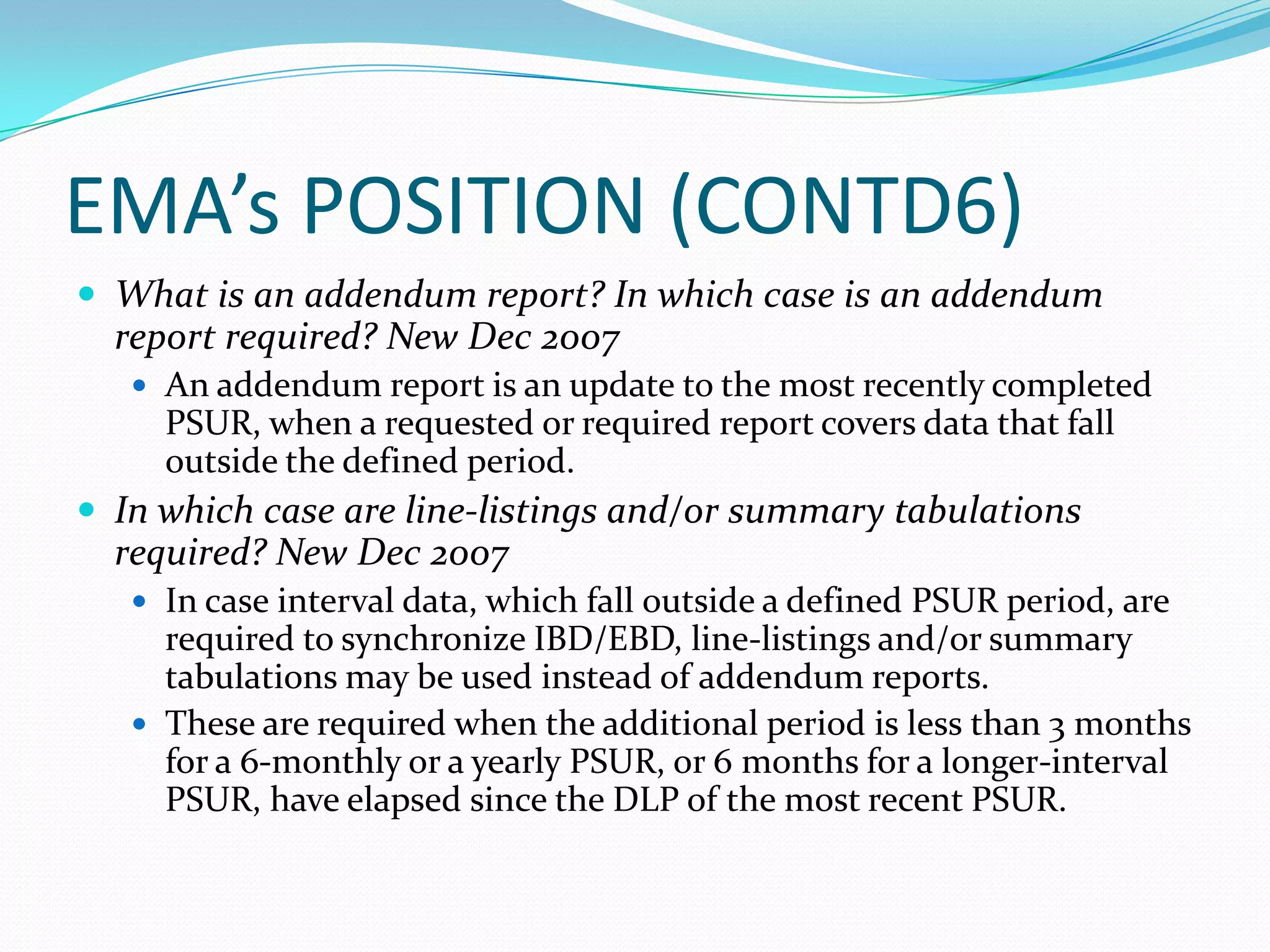
Periodic Safety Update Reports (PSURs) are pharmacovigilance documents required from marketing authorization holders (MAHs) to evaluate the risk-benefit balance of medicinal products post-authorization. They must be submitted at specified intervals, based on EU regulations, using data from various sources to assess new safety information and overall safety evaluations. The PSUR must include sections such as executive summaries, worldwide marketing authorizations, patient exposure, and discussions about efficacy and safety findings.

![What is PSUR?
Essentials:
Periodic safety update reports (PSURs) are pharmacovigilance documents intended to provide an evaluation of the risk-benefit
balance of a medicinal product for submission by marketing authorisation holders (MAHs) in the light of new or changing
information at defined time points during the post-authorisation phase. The required format and content of PSURs in the EU
are based on those for the Periodic Benefit Risk Evaluation Report (PBRER) described in the ICH-E2C(R2) guideline. The PBRER
replaces the PSUR format previously described in the ICH-E2C(R1). In the EU, the report shall be described and named as PSUR [IR
Art 34 and 35].
Time:
They are submitted regularly with periodicity established in Regulation
(EC) No 726/2004 and Directive 2001/83/EC
Time 6m 1y 1,5y 2y 3y 4y 7y
Authorisation
& Market
!!!NO MATTER IF IT IS MARKETED OR NOT MARKETED!!! YOU
SHALL SUBMIT!!!
Where:
They should be submitted to the Competent Authorities of all Member
States and to the Agency if products are authorised through centralised
procedure, or only to the Competent Authorities if they are authorised
nationally](https://image.slidesharecdn.com/psurkkch1tampl-120926015213-phpapp02/75/PSUR-Requirements-2-2048.jpg)