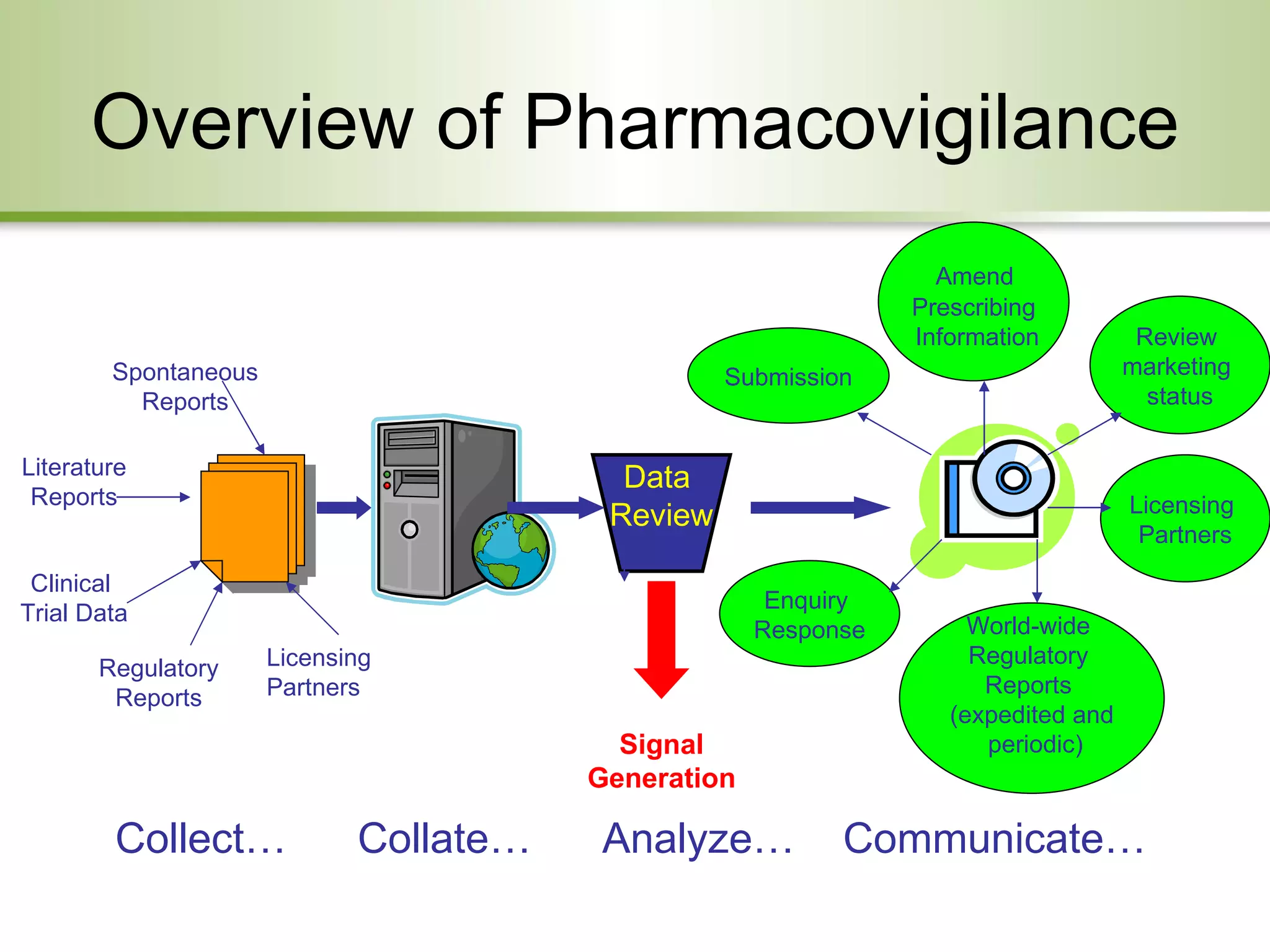
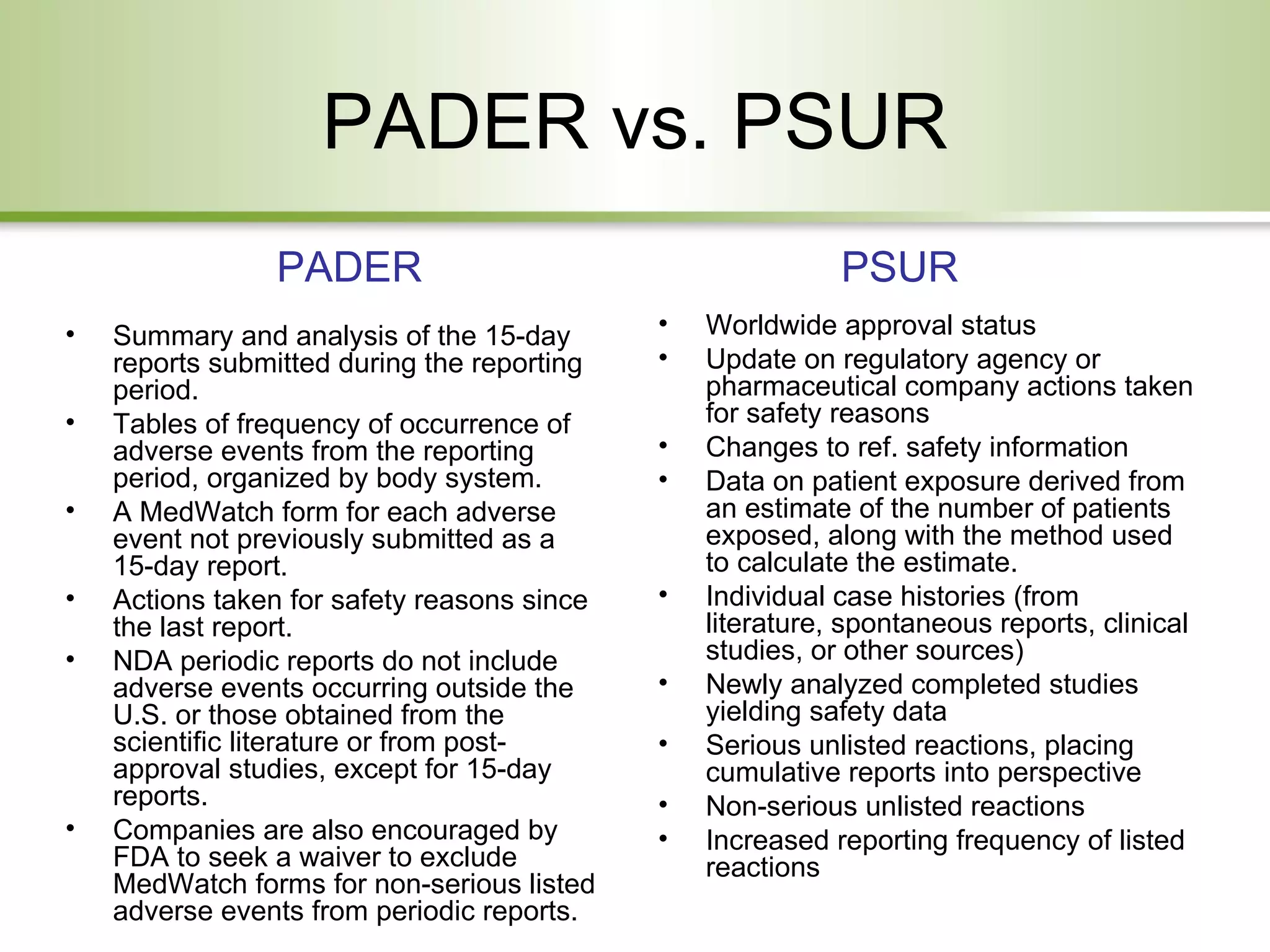
This document provides an overview of pharmacovigilance systems and regulations in the US and EU. It describes regulatory oversight bodies, key regulations governing pharmacovigilance, safety reporting requirements during pre-marketing and post-marketing periods, pediatric legislation differences, and risk management strategies between the regions.