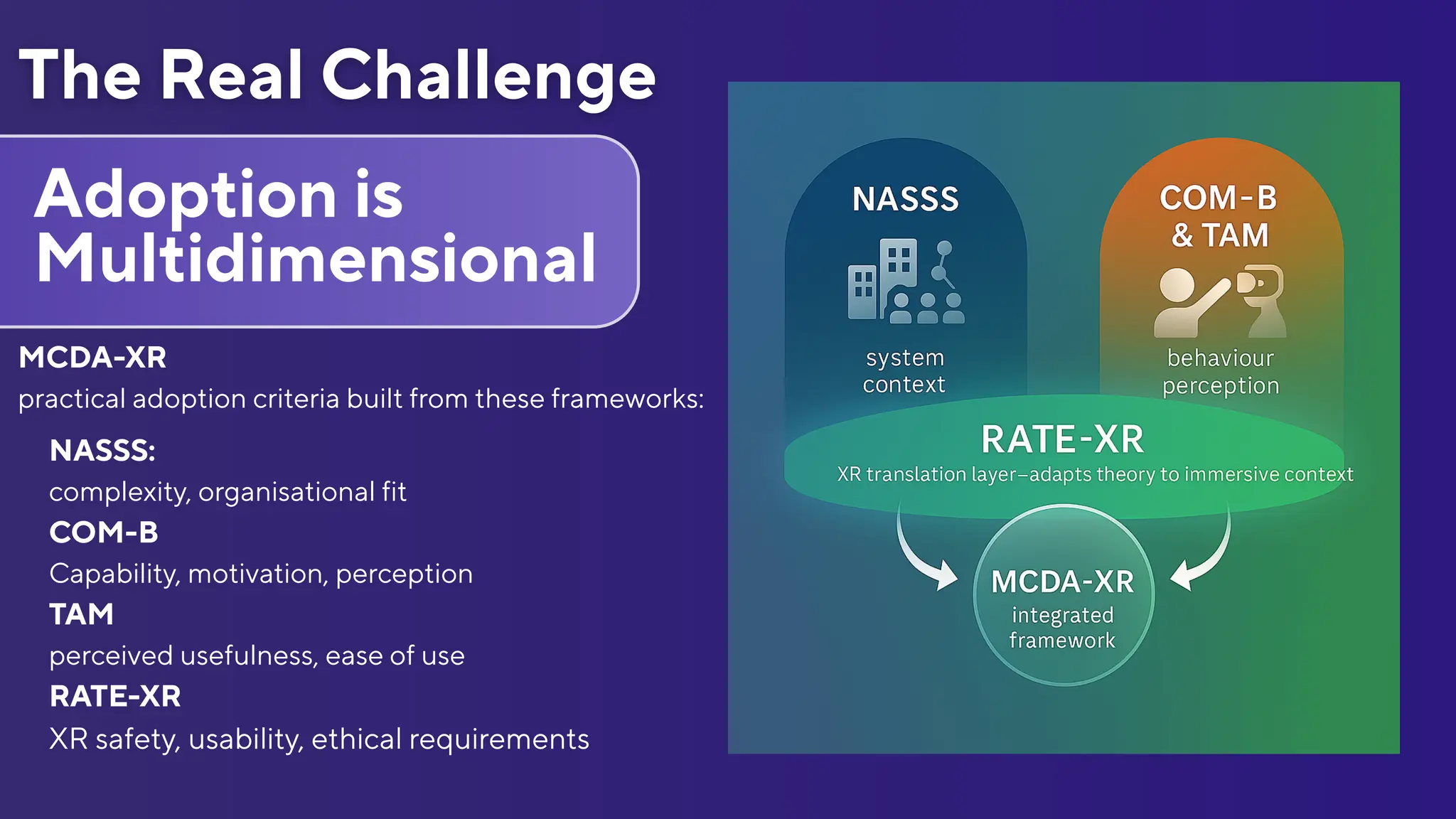
Document que va acompanyar la intervenció del panel d'experts que va parlar el 8 de desembre de 2025 a United XR Europe-Stereopsia sobre com assegurar la supervivència dels projectes de realitat ampliada més enllà de la recerca i com fer que formin part de la pràctica clínica real. El metge d'Innovació de Badalona Serveis Assistencials José Ferrer era un dels components del panel. L'esdeveniment United XR Europe-Stereopsia es va celebrar a Brusel·les.