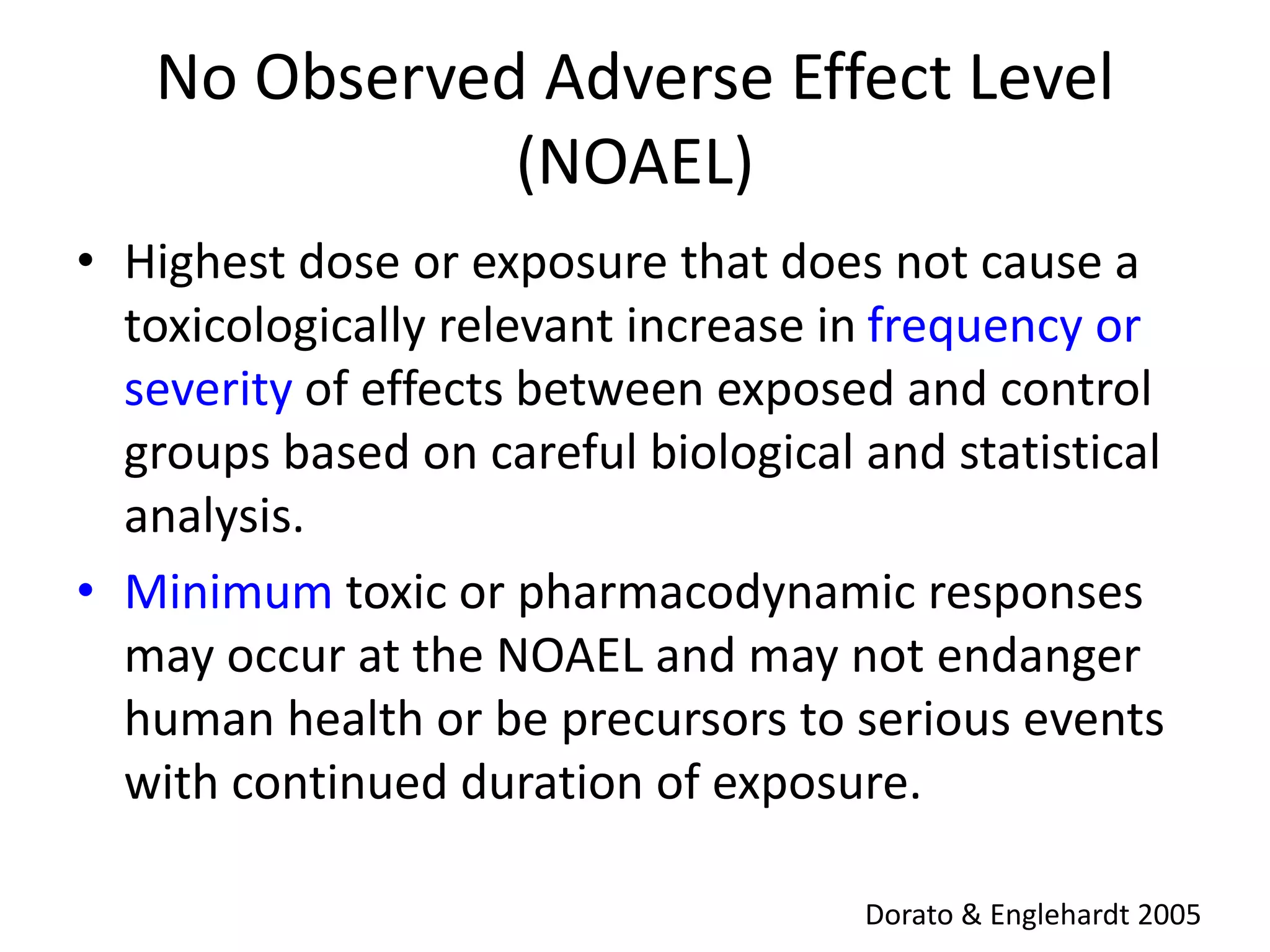
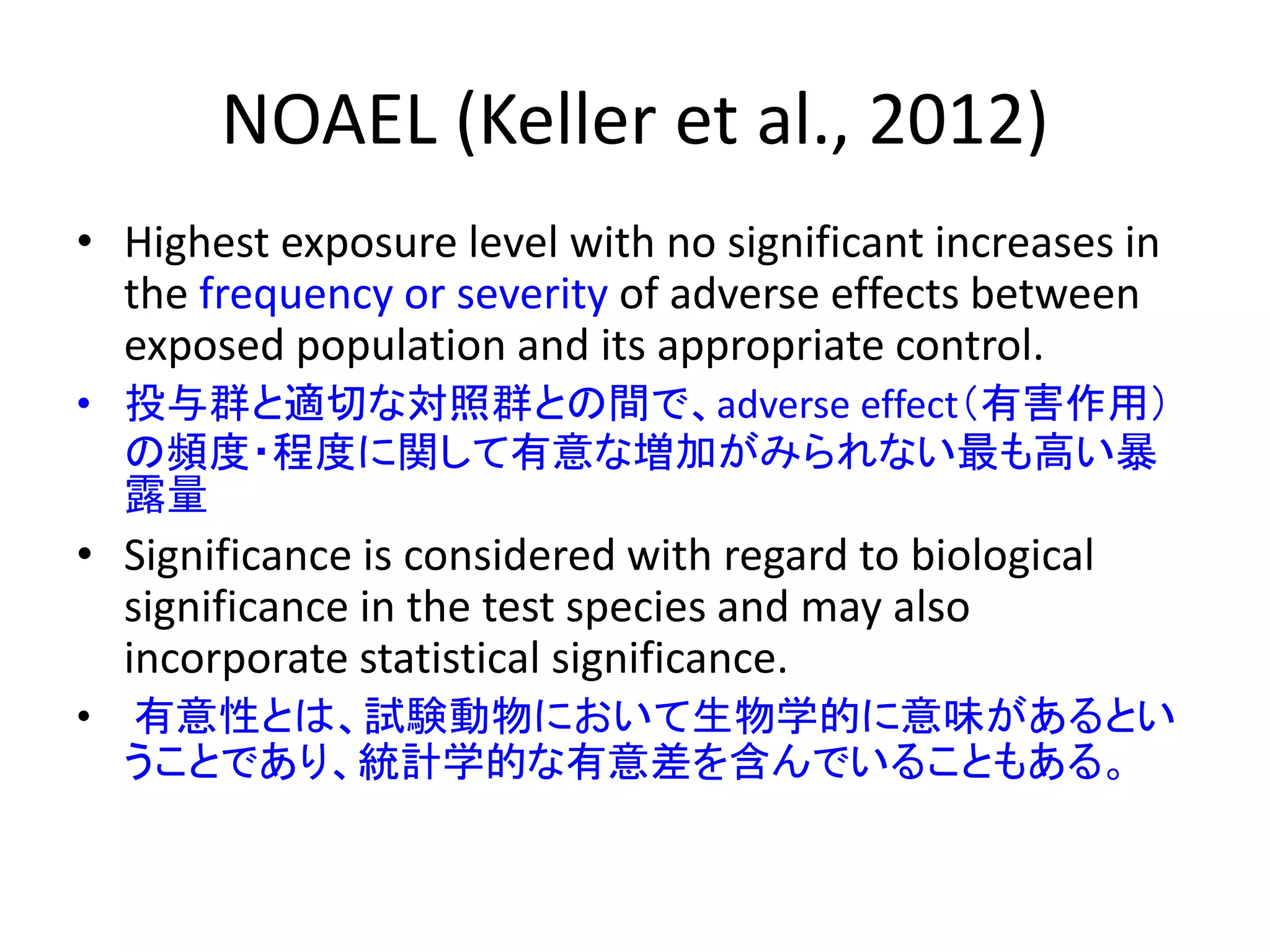
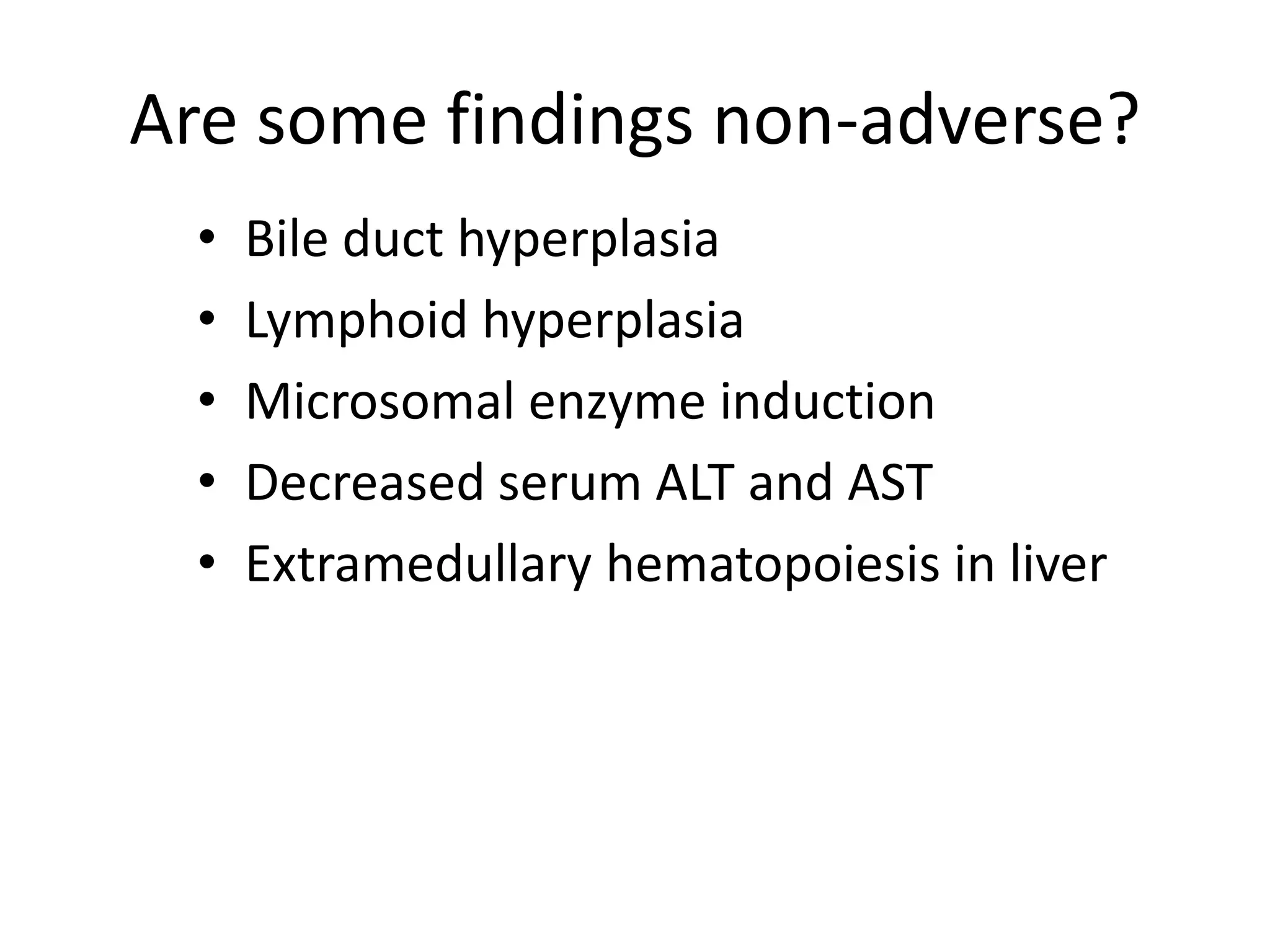
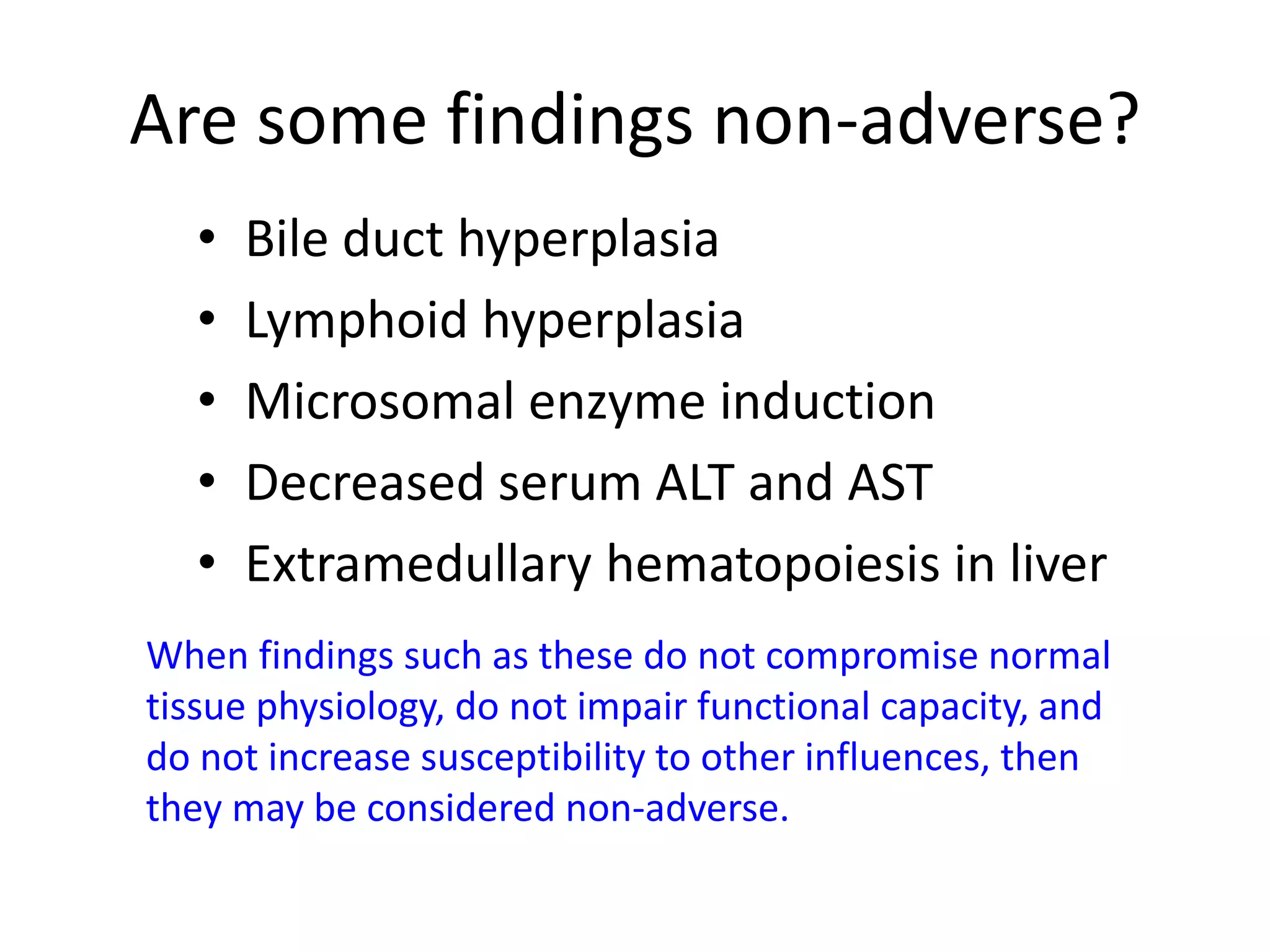
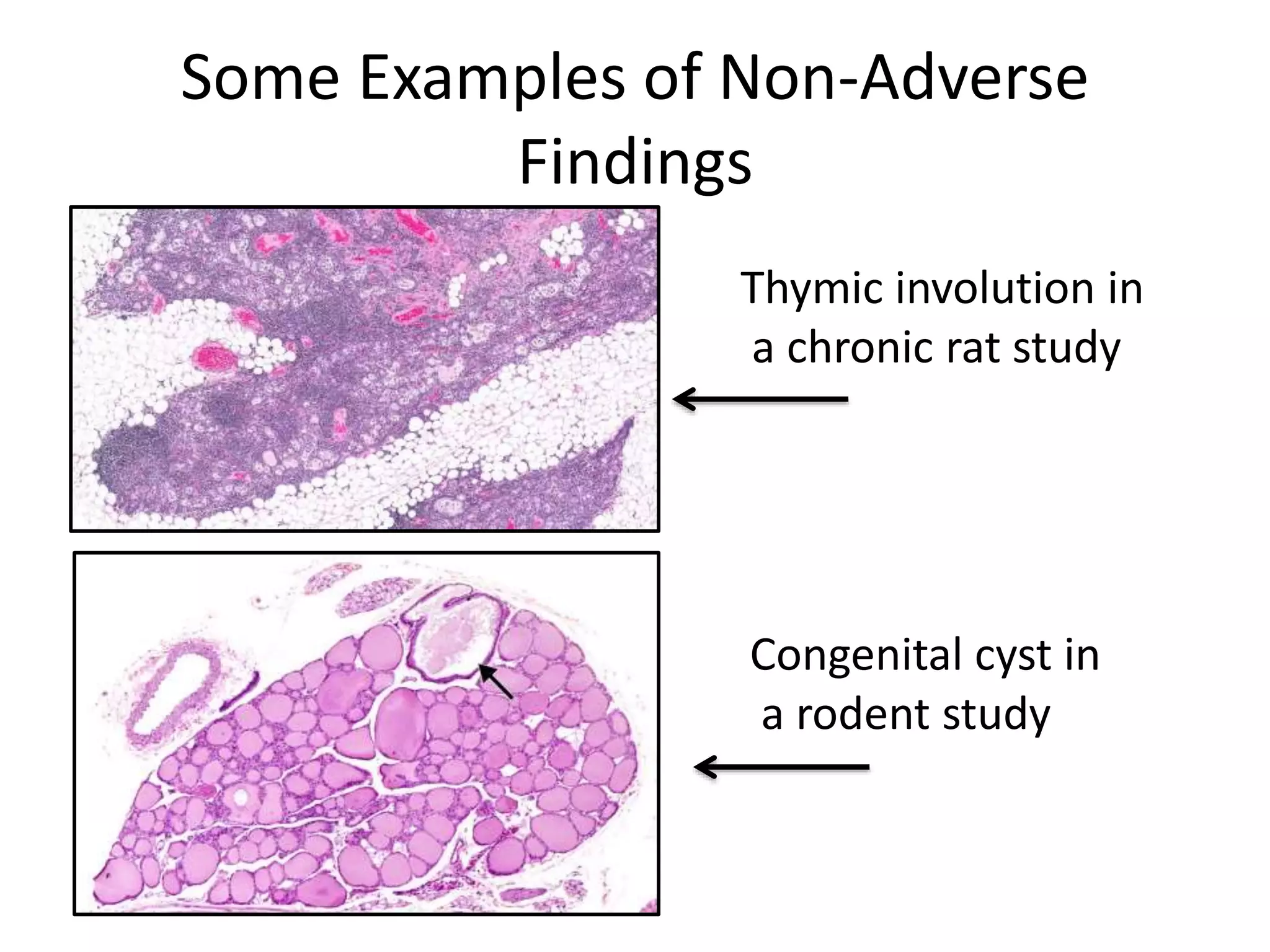
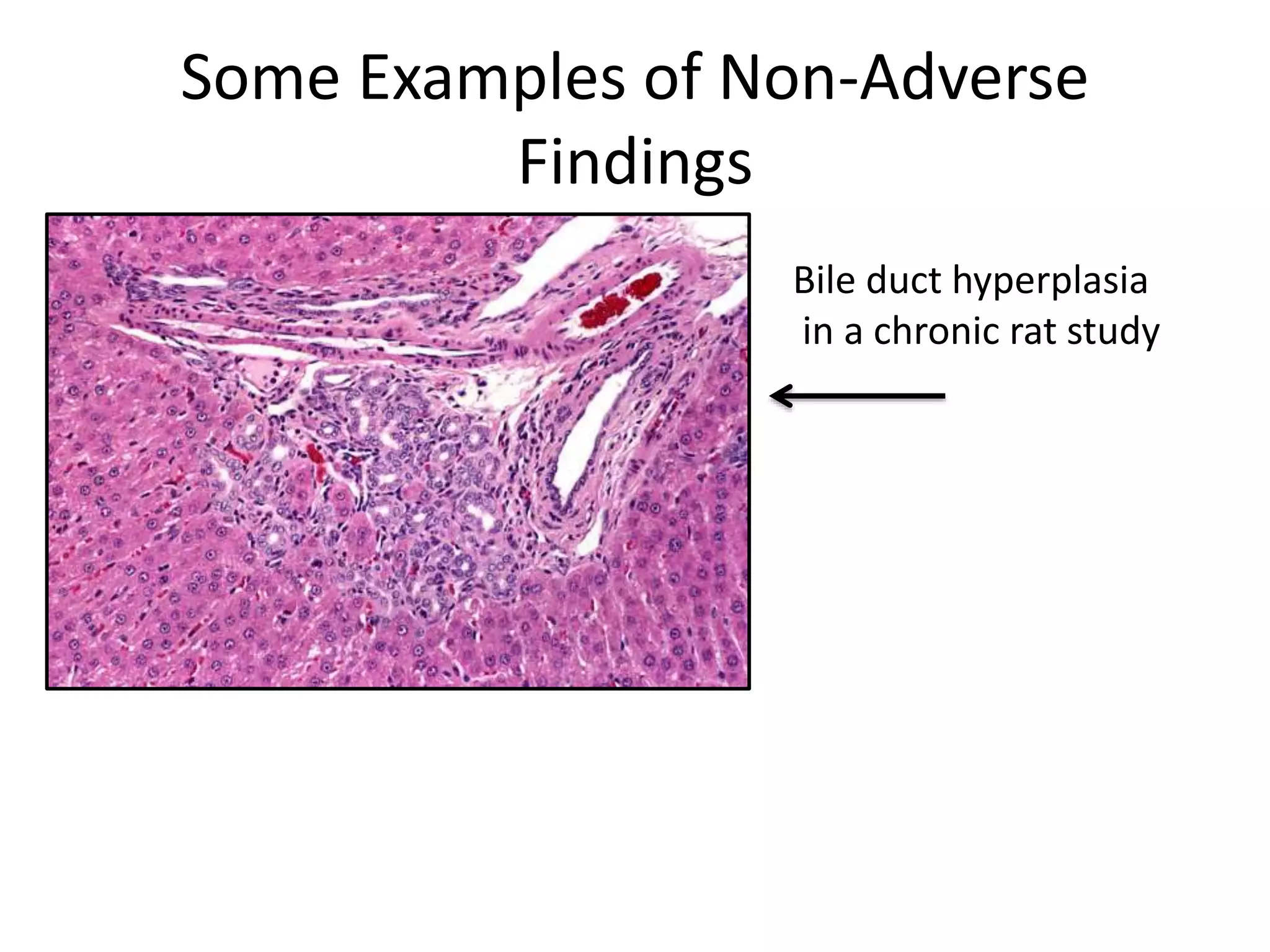
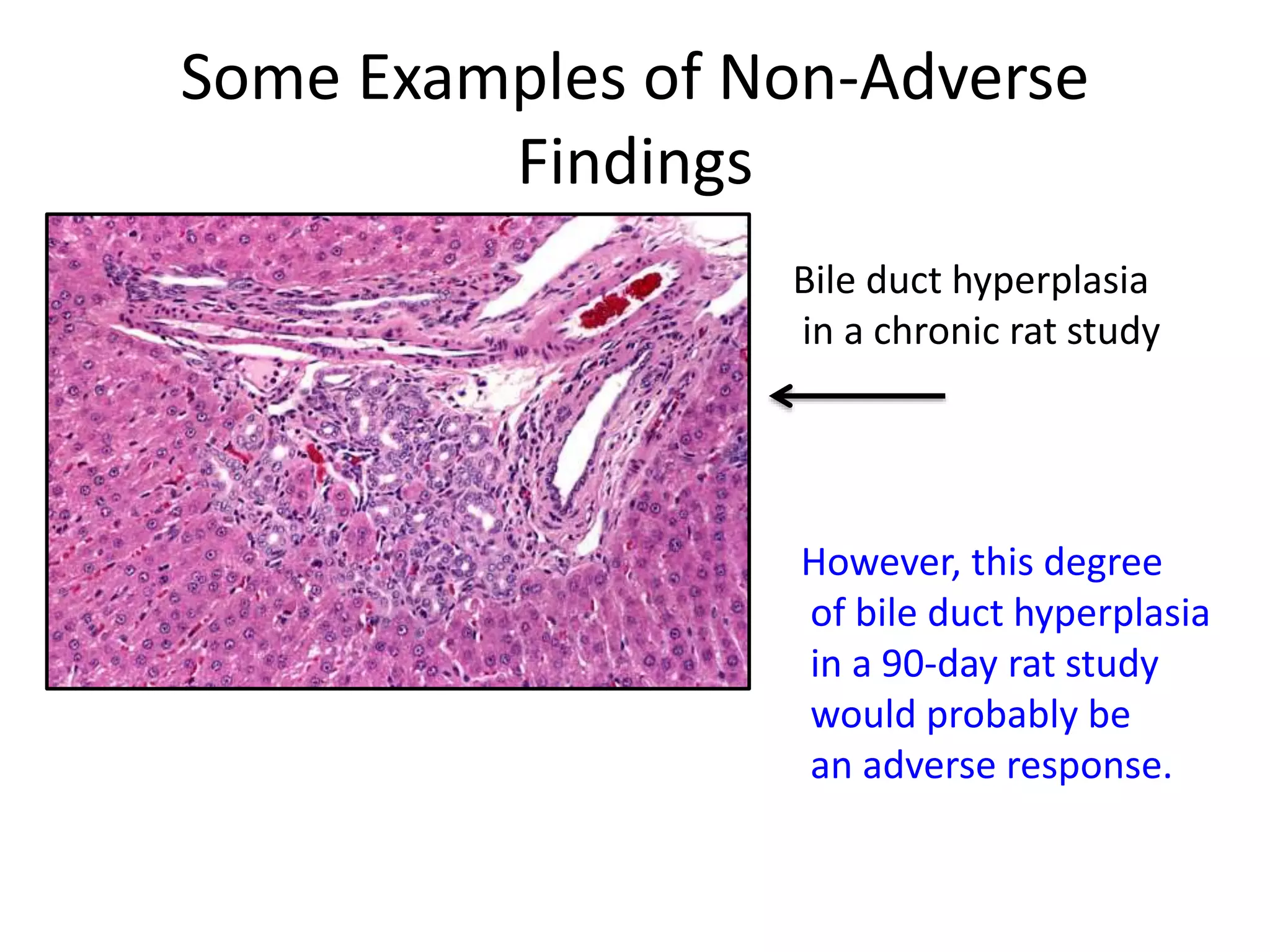
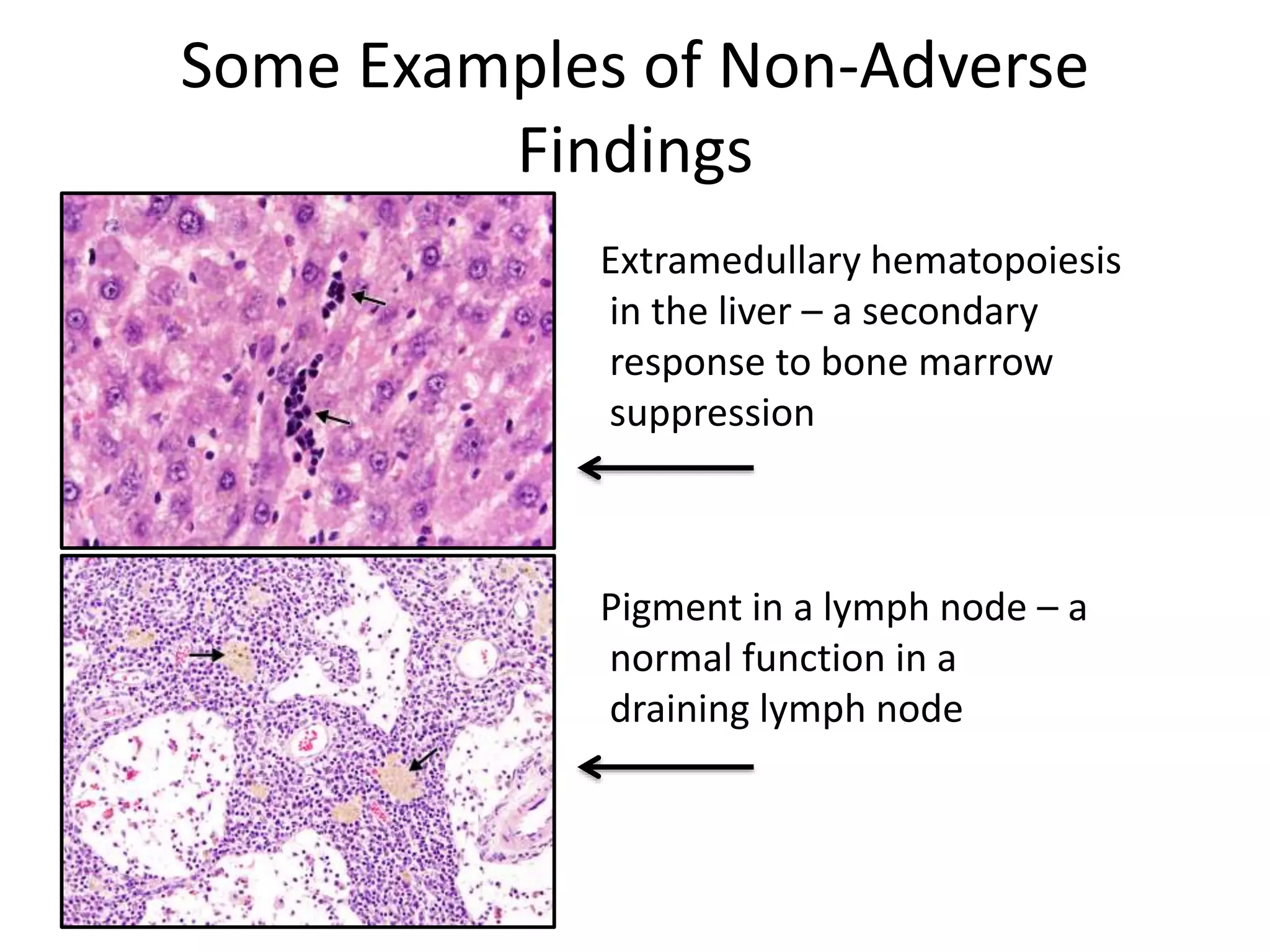
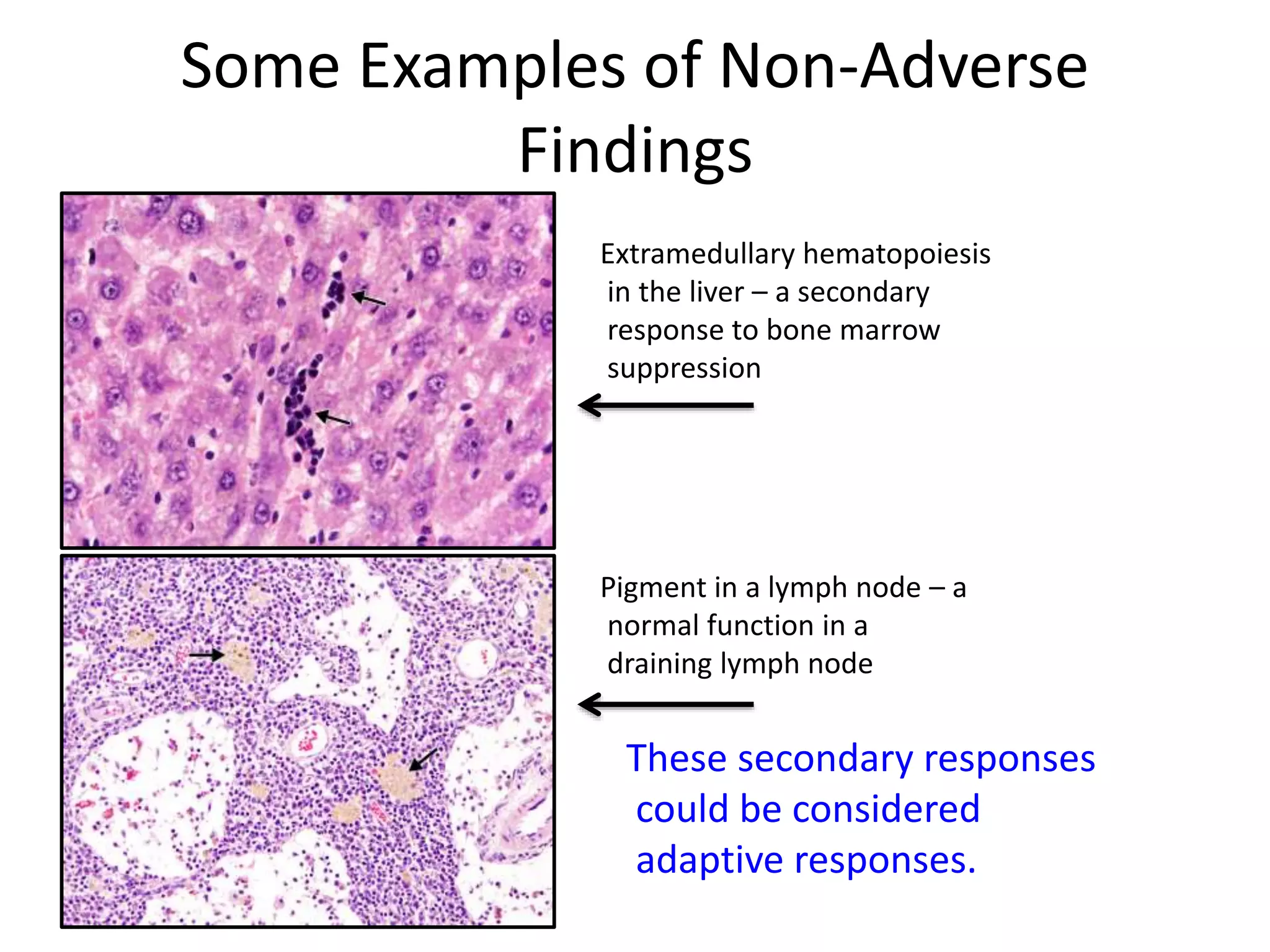
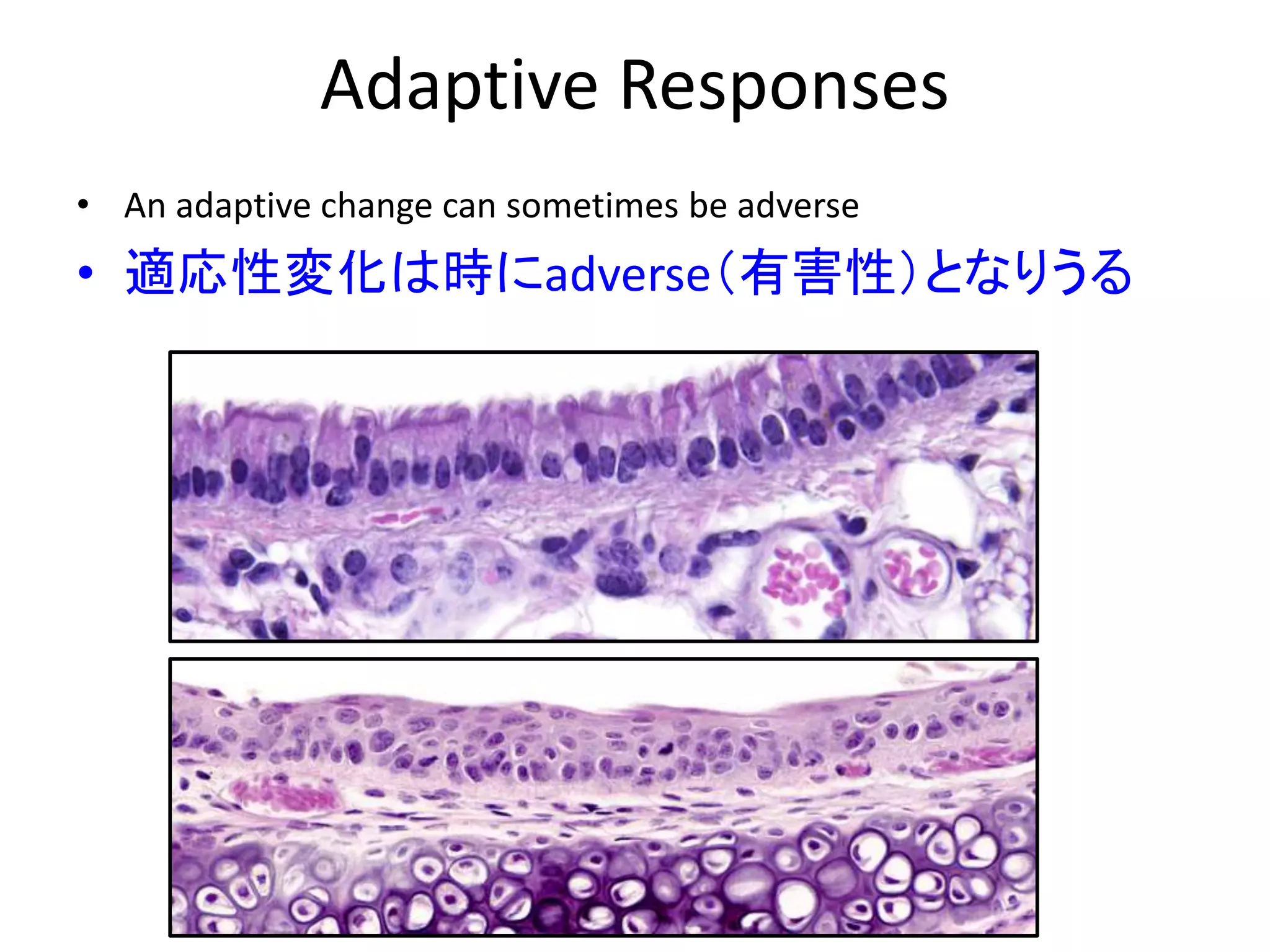
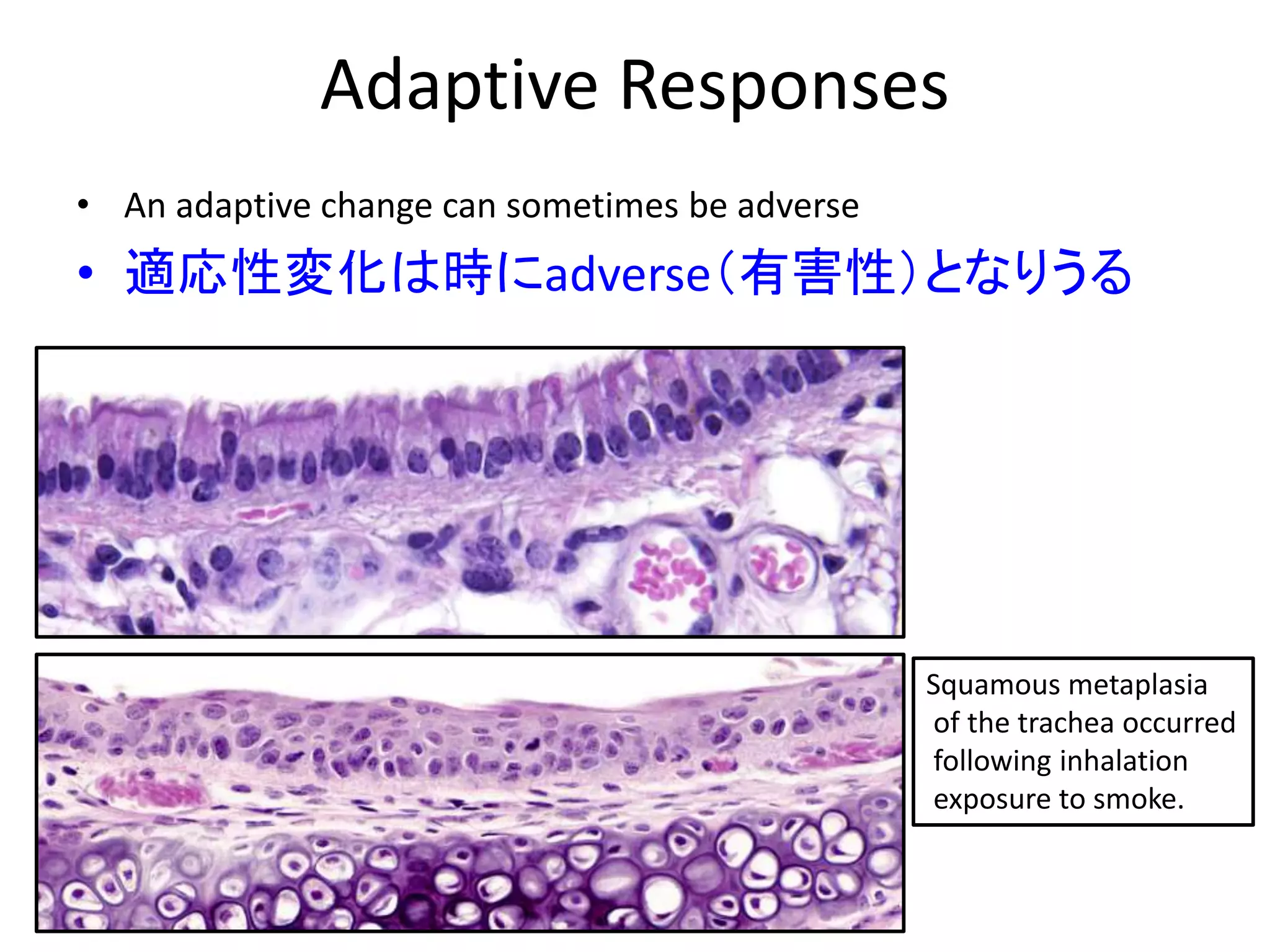
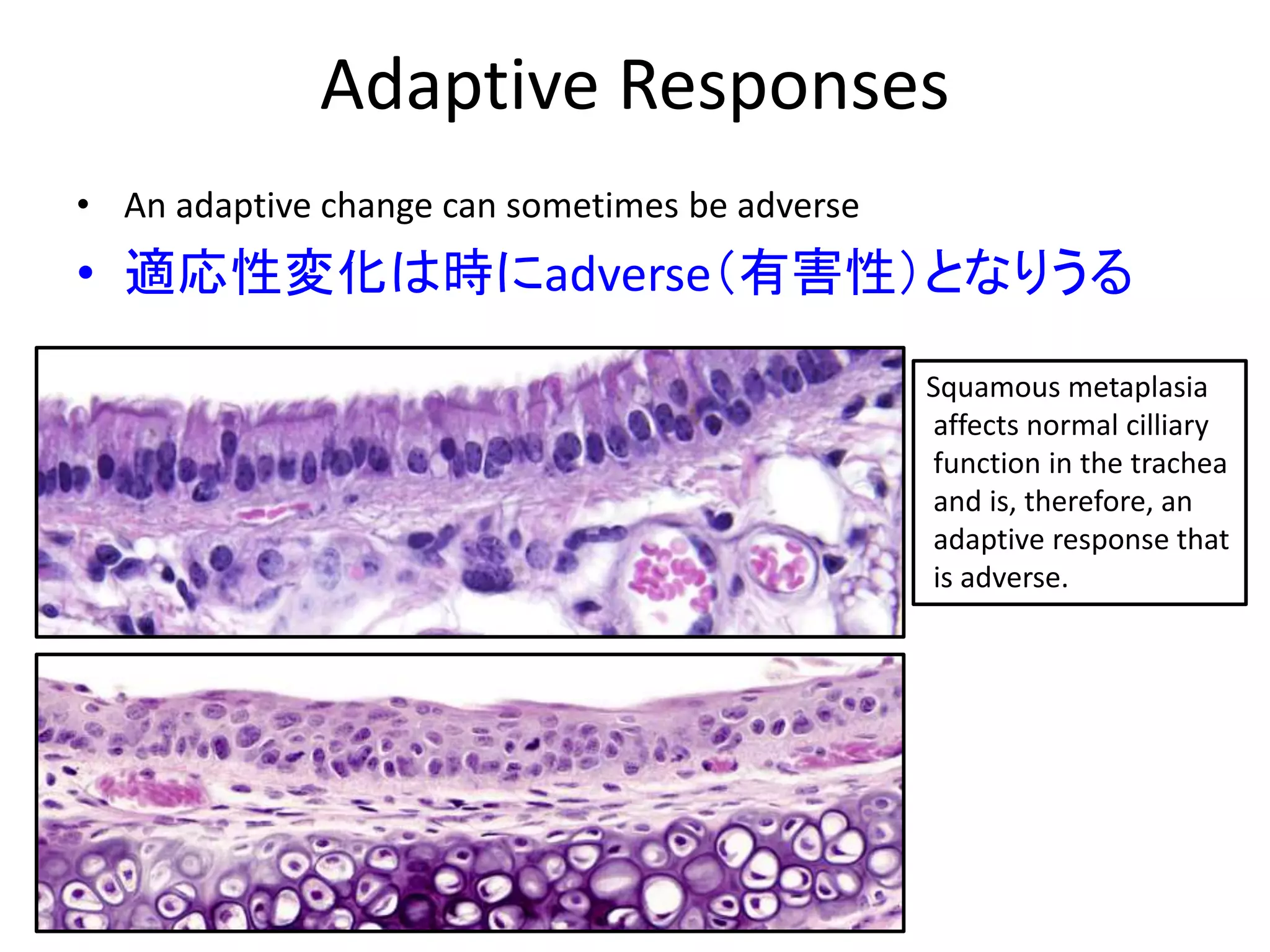
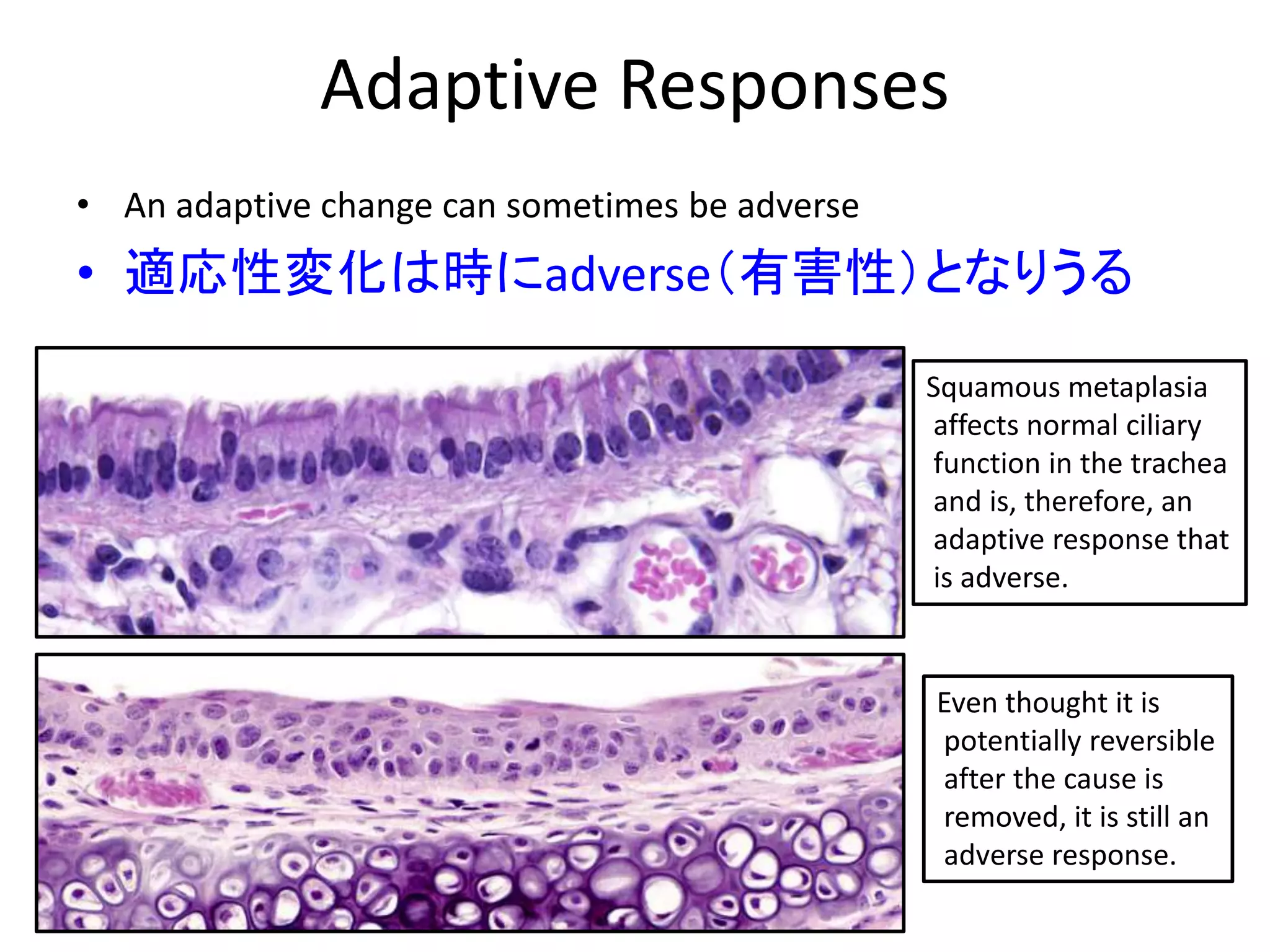
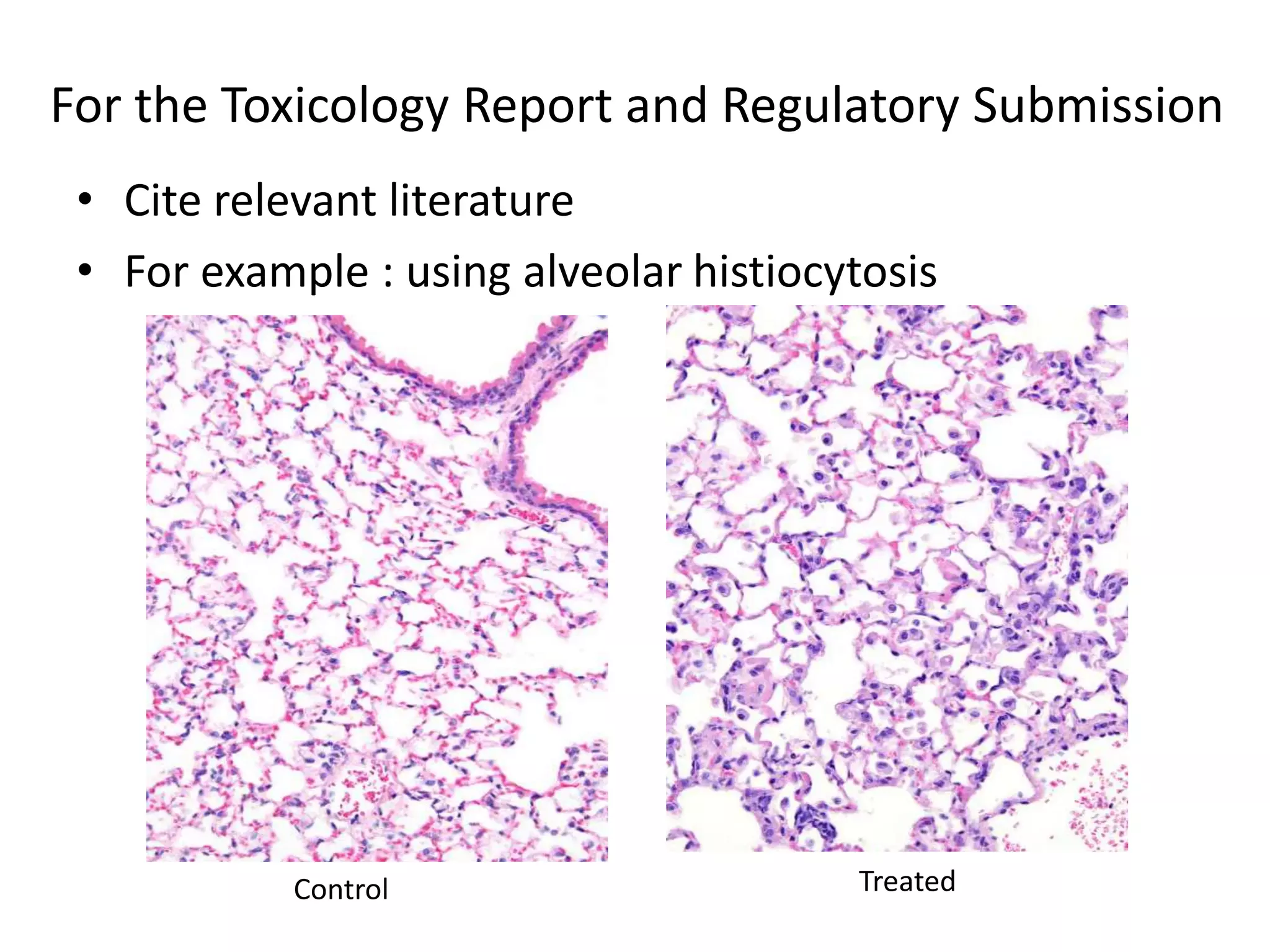
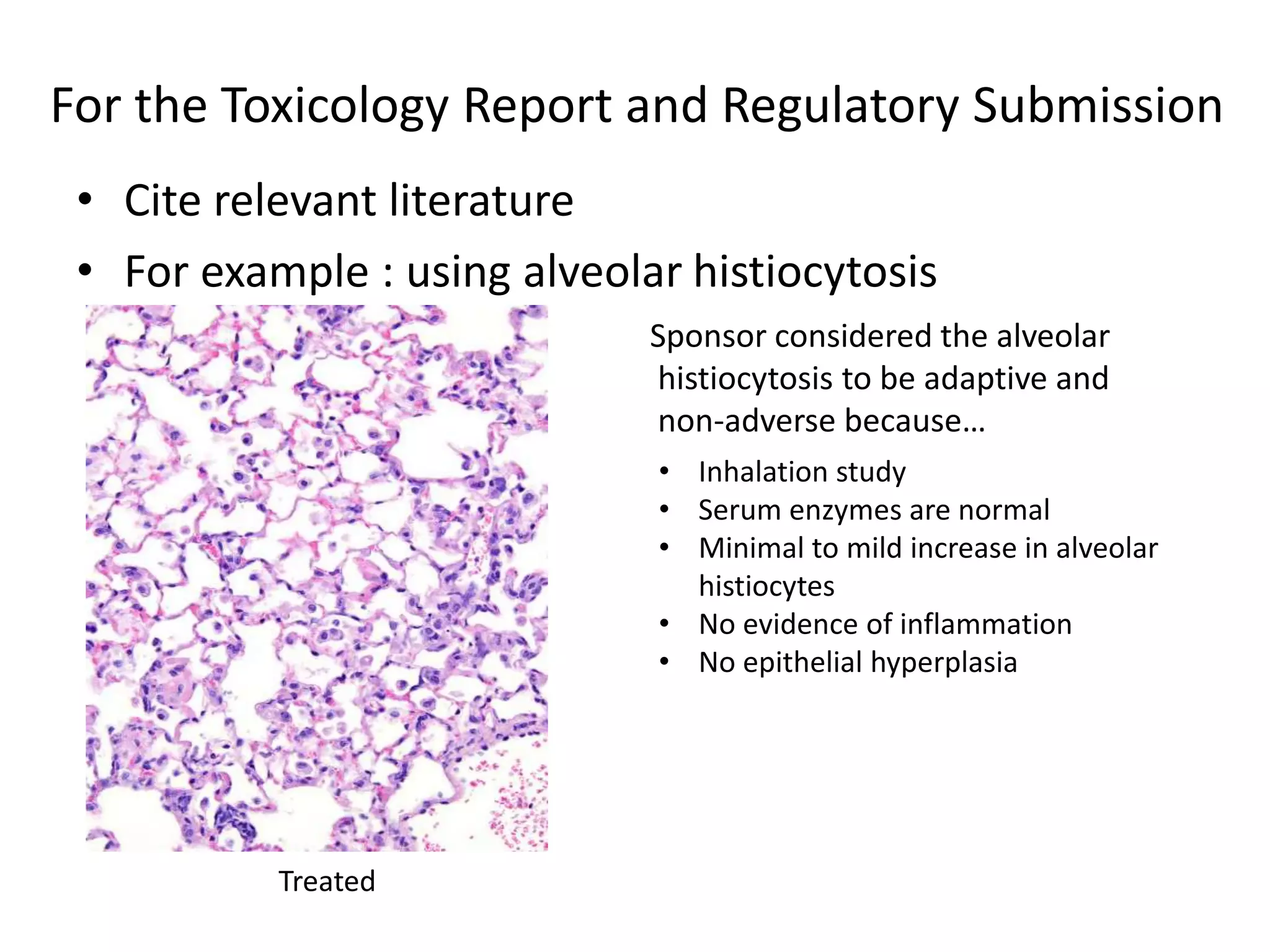
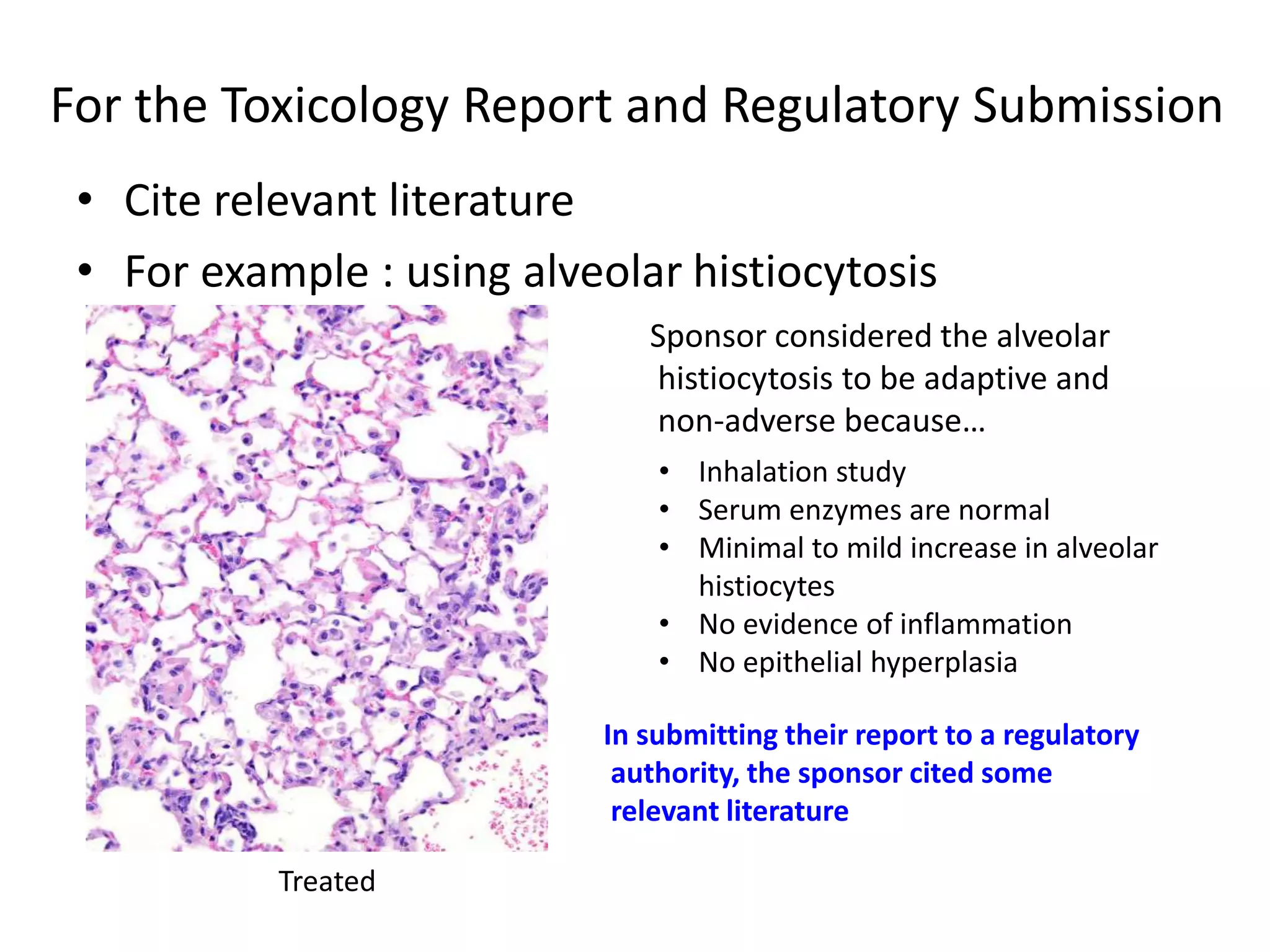
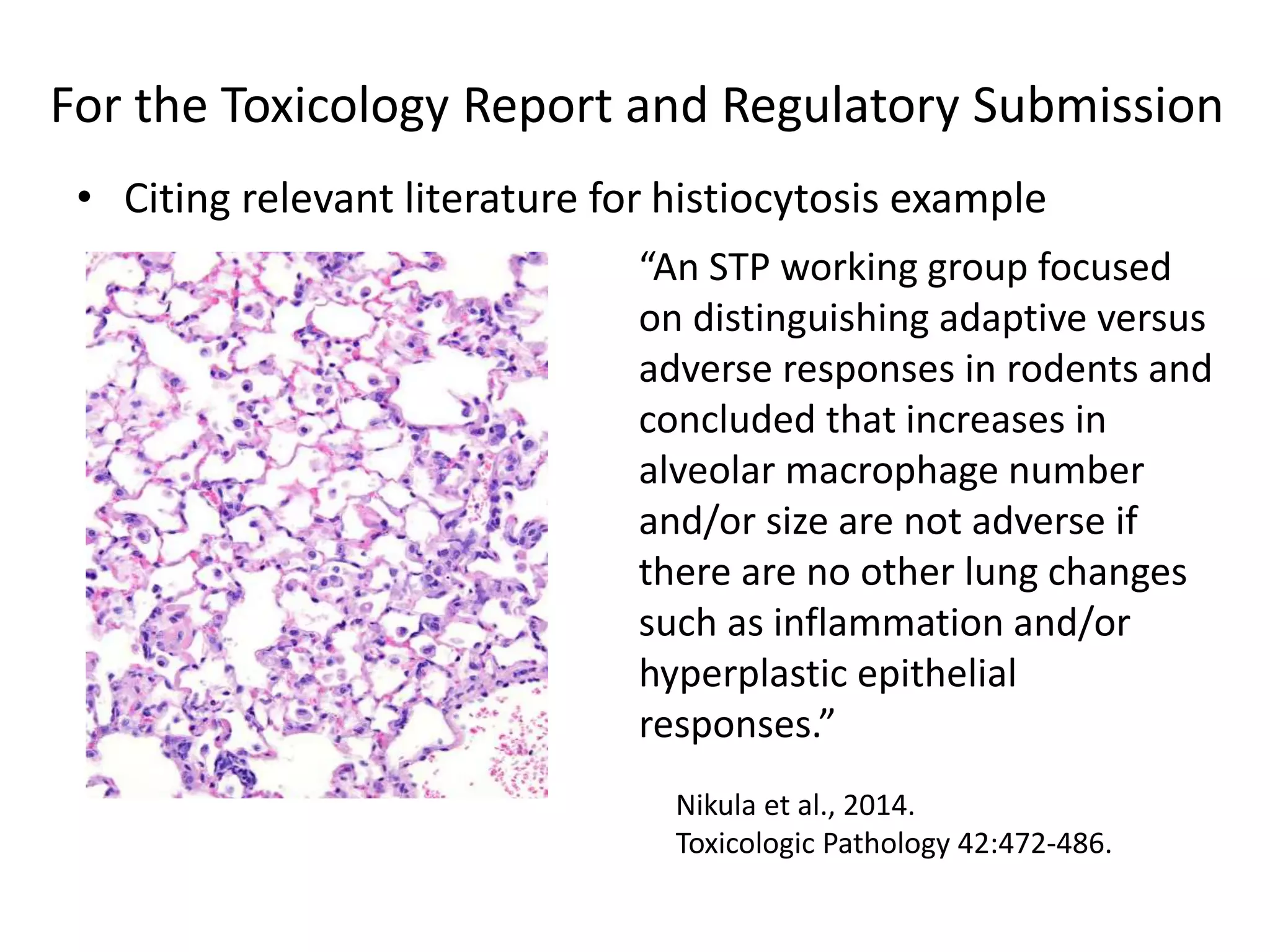
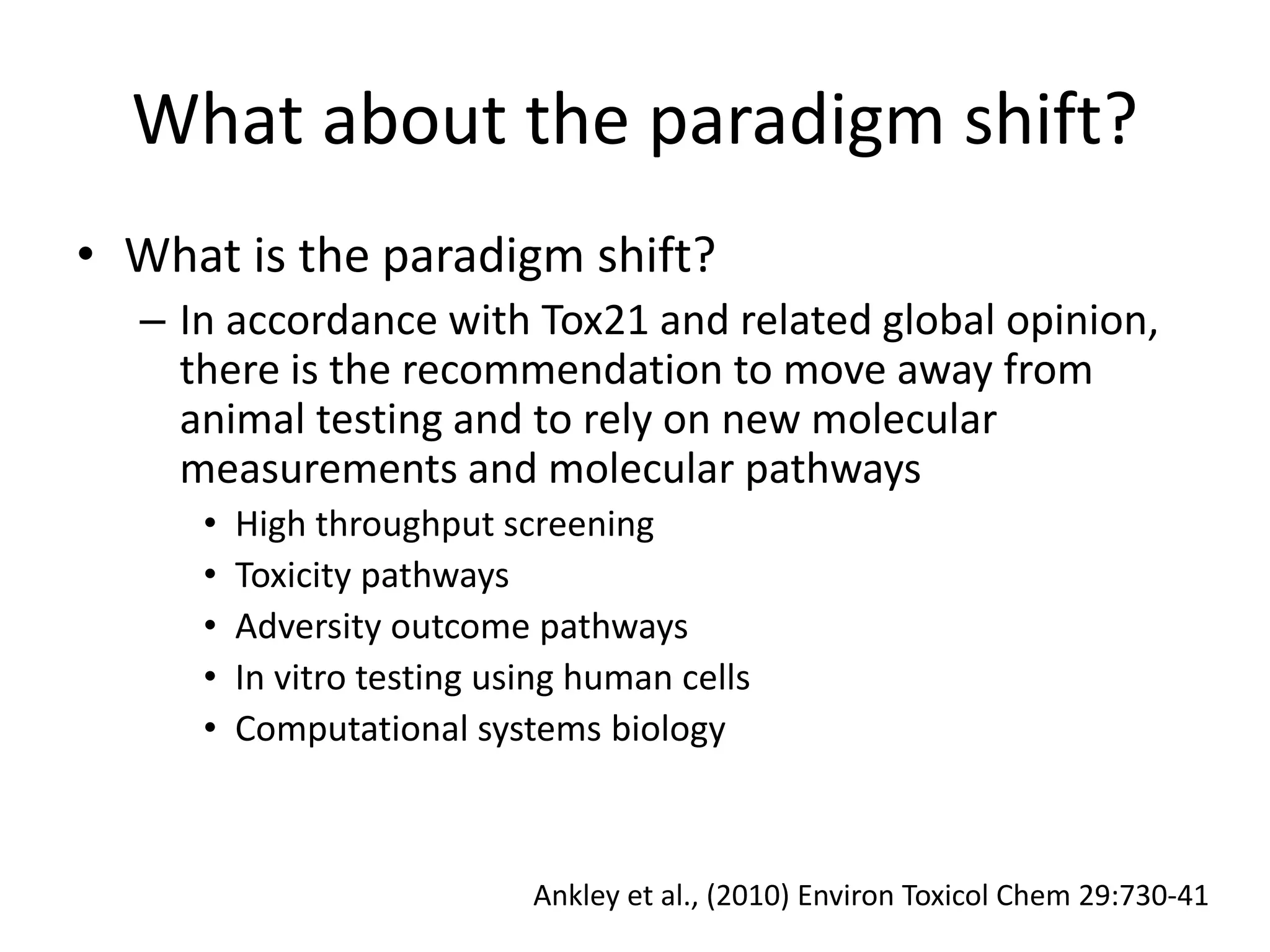
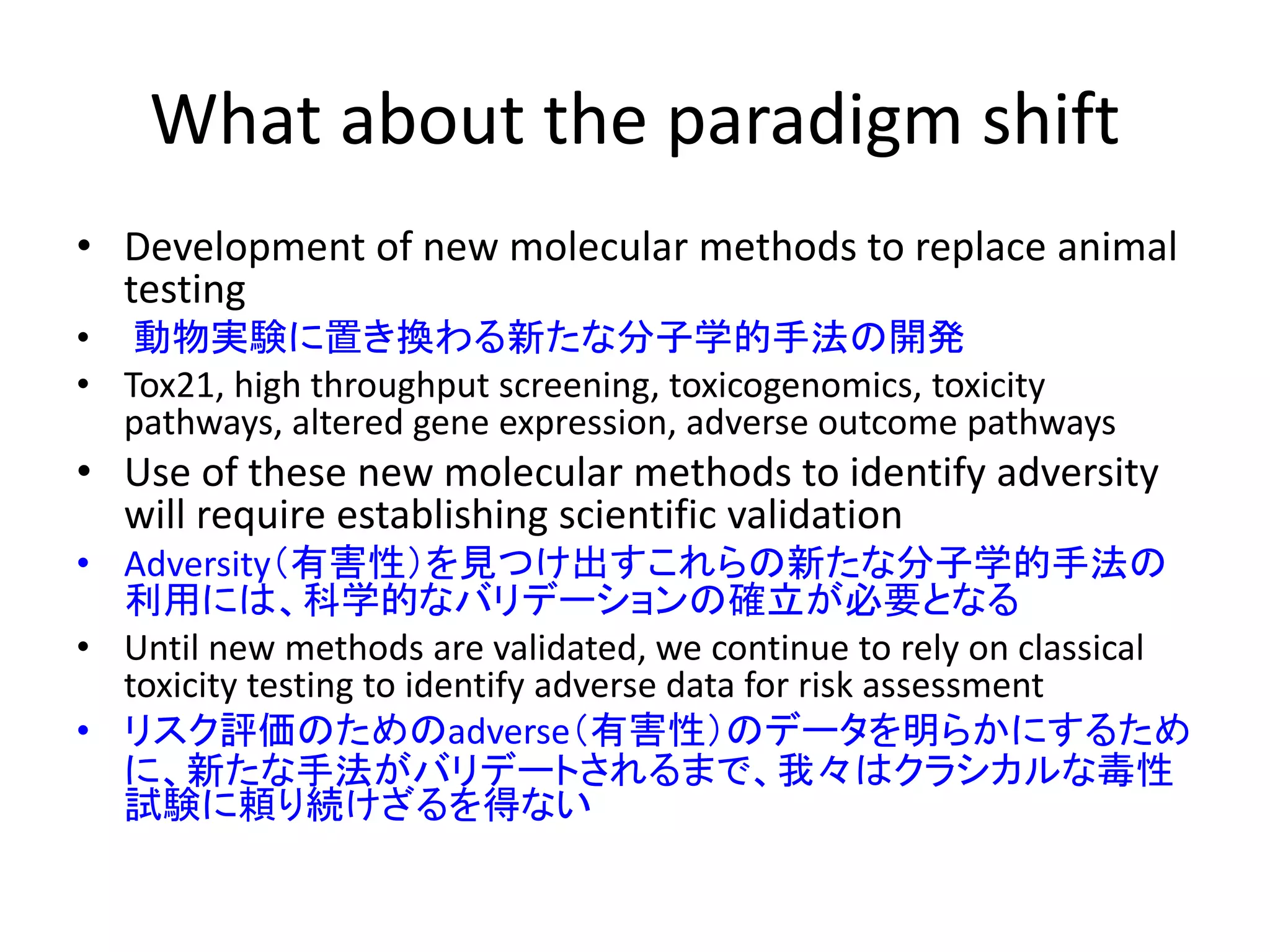
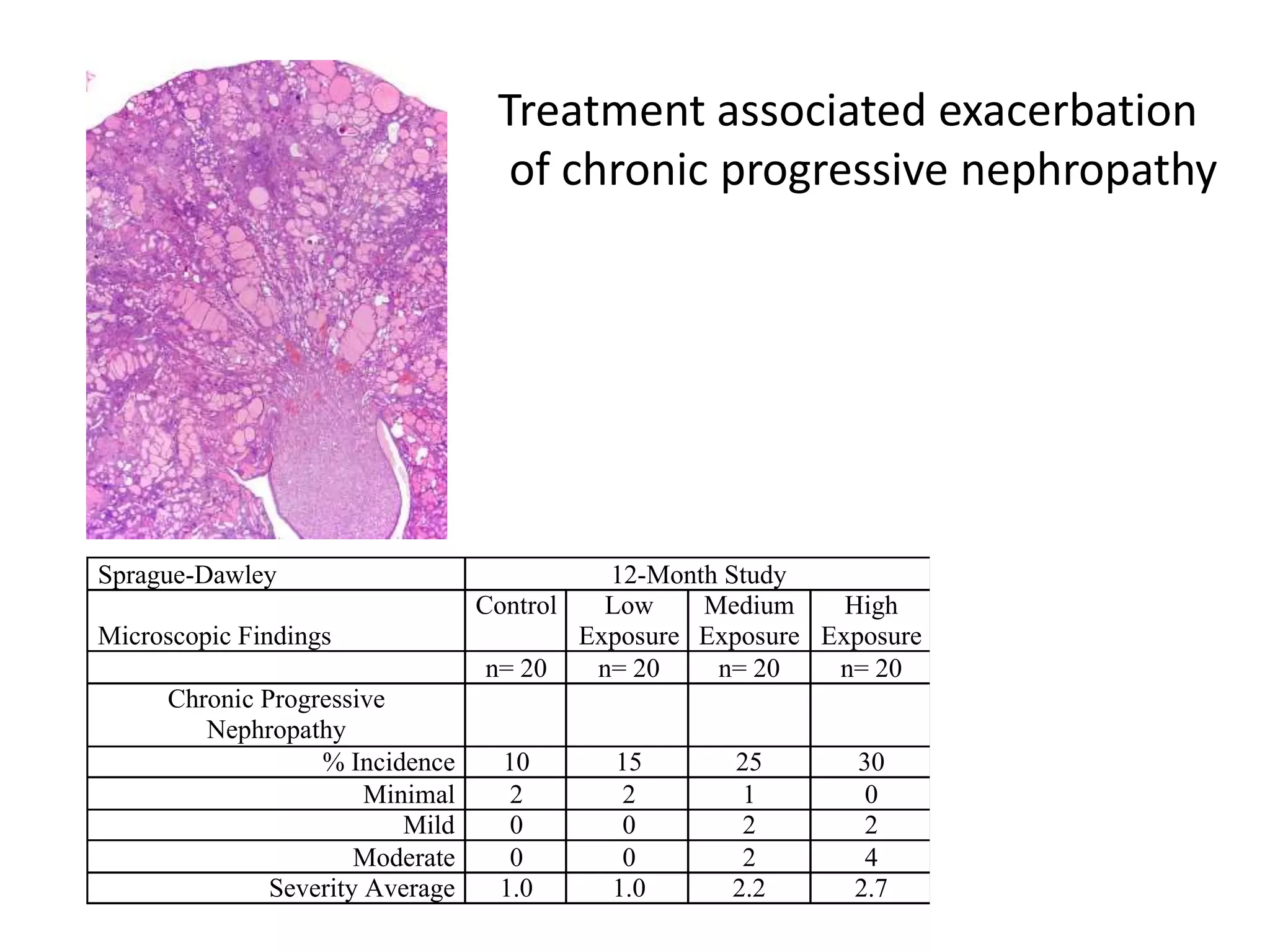
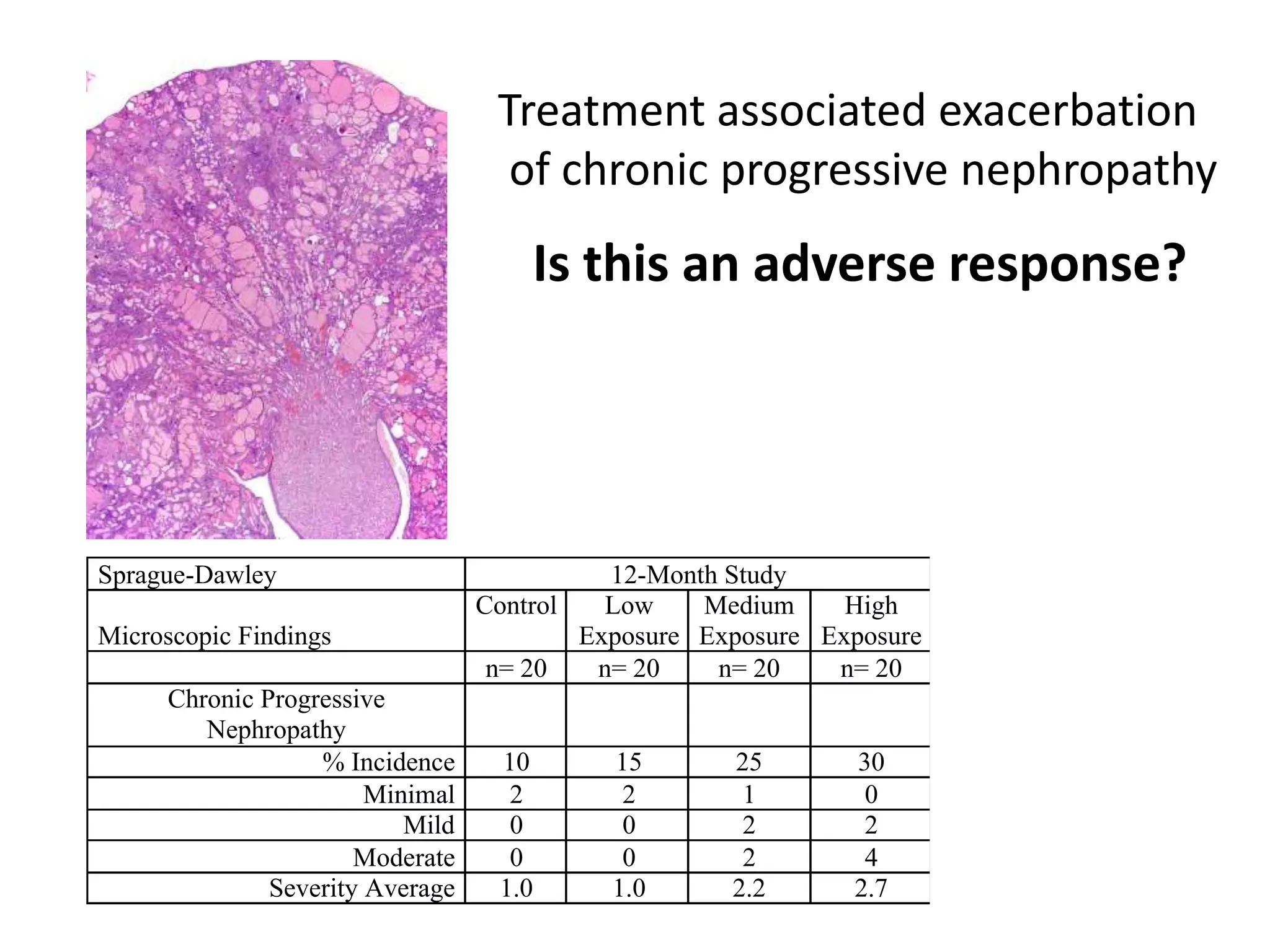
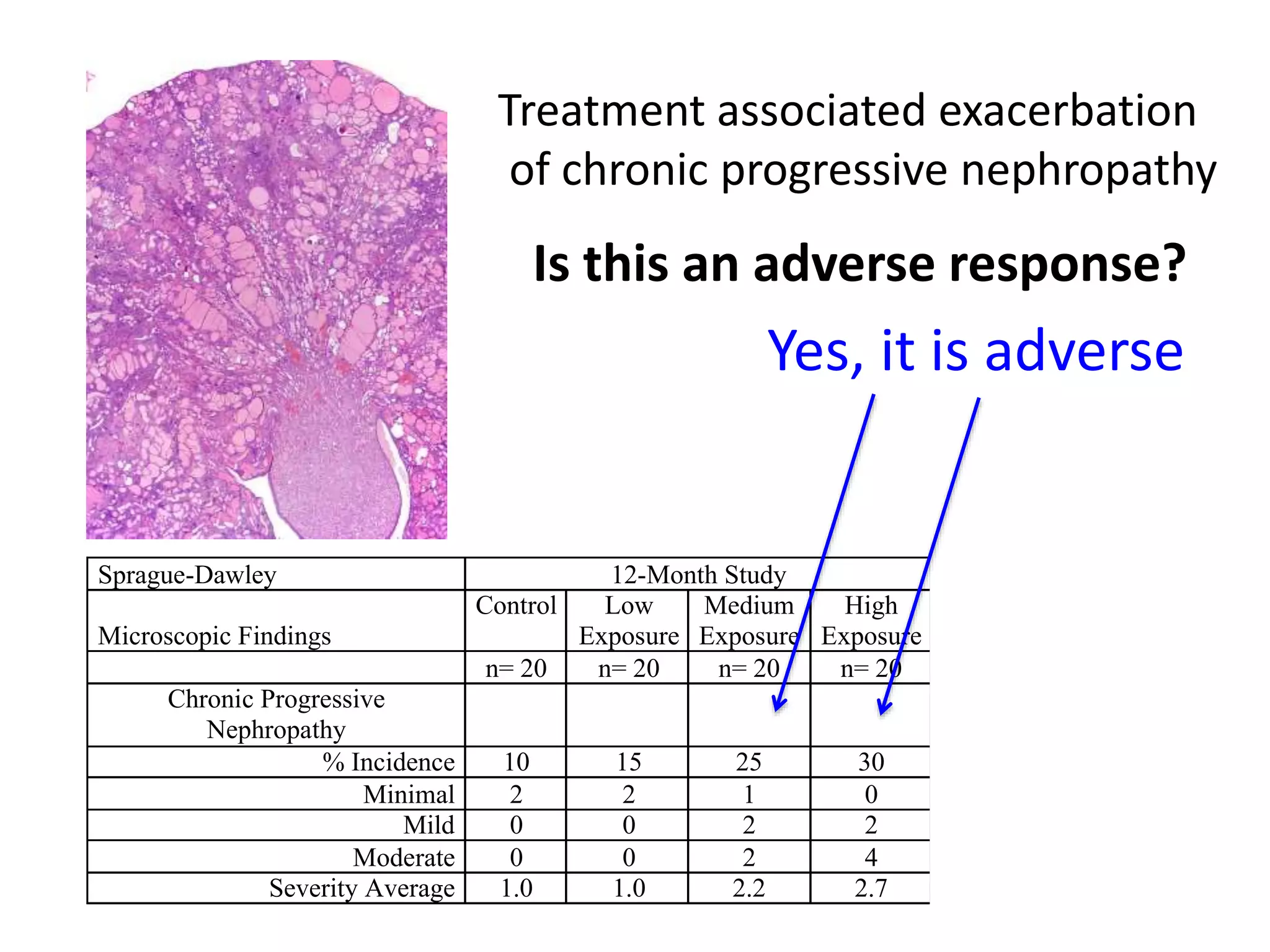
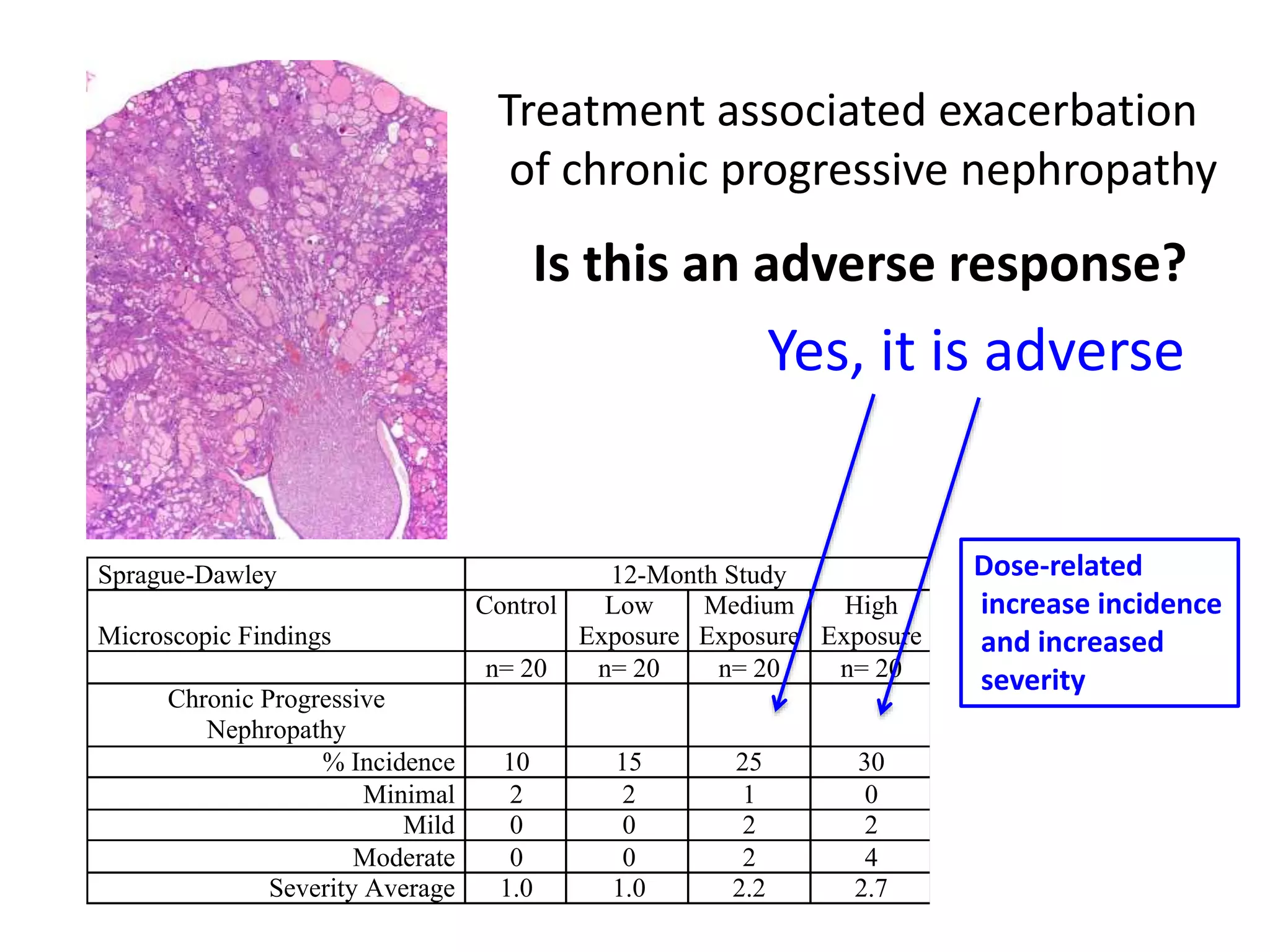
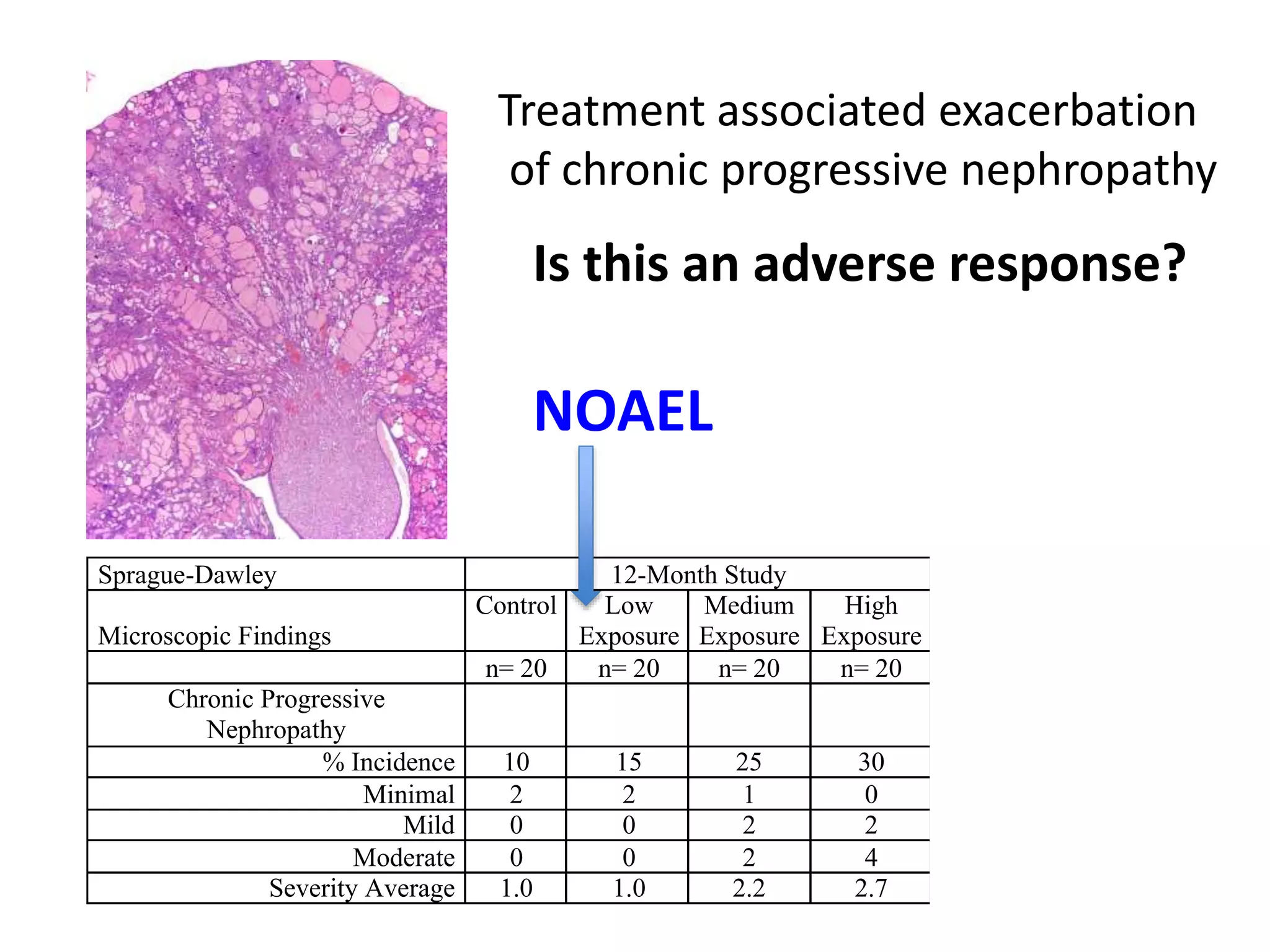
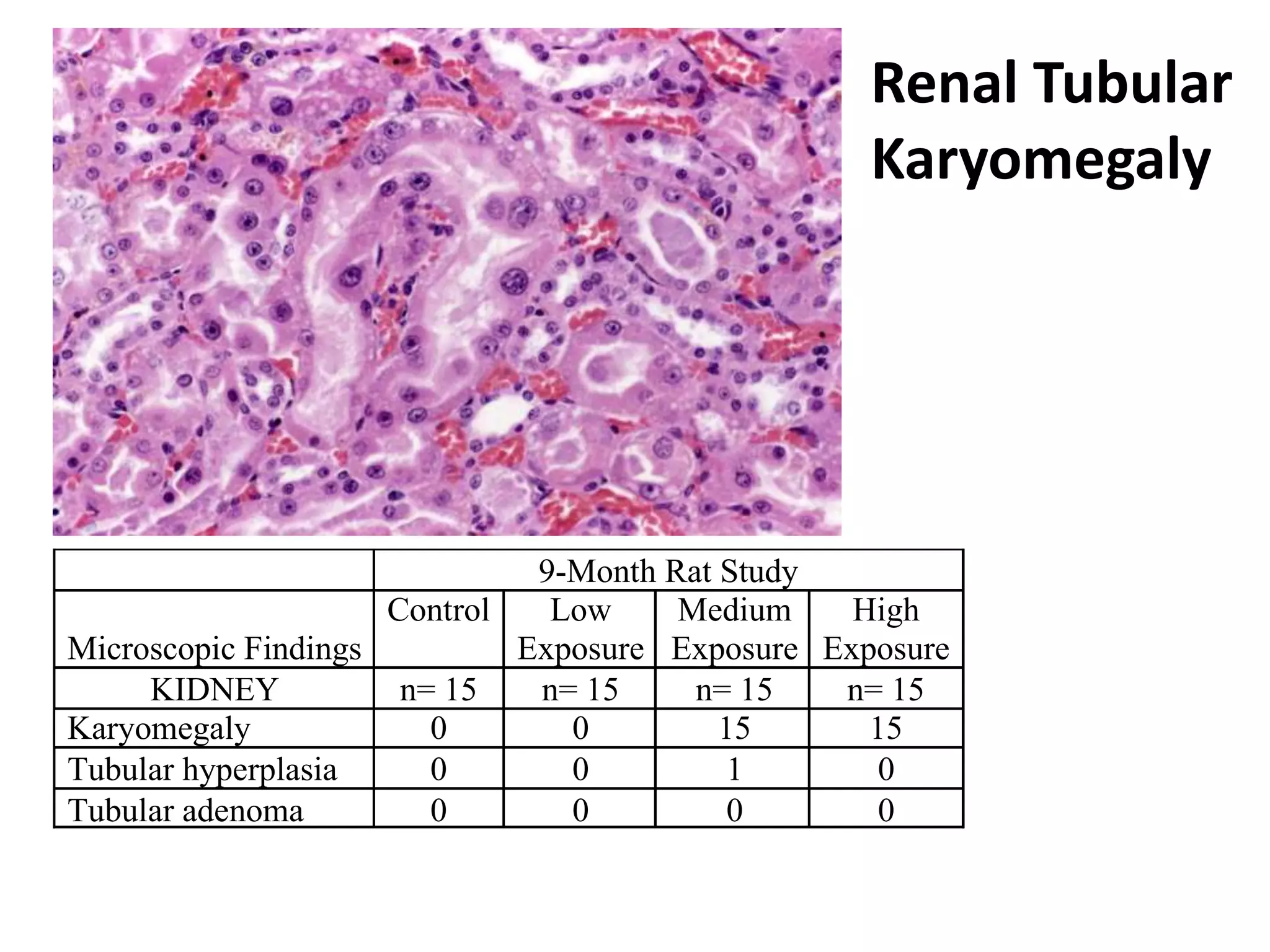
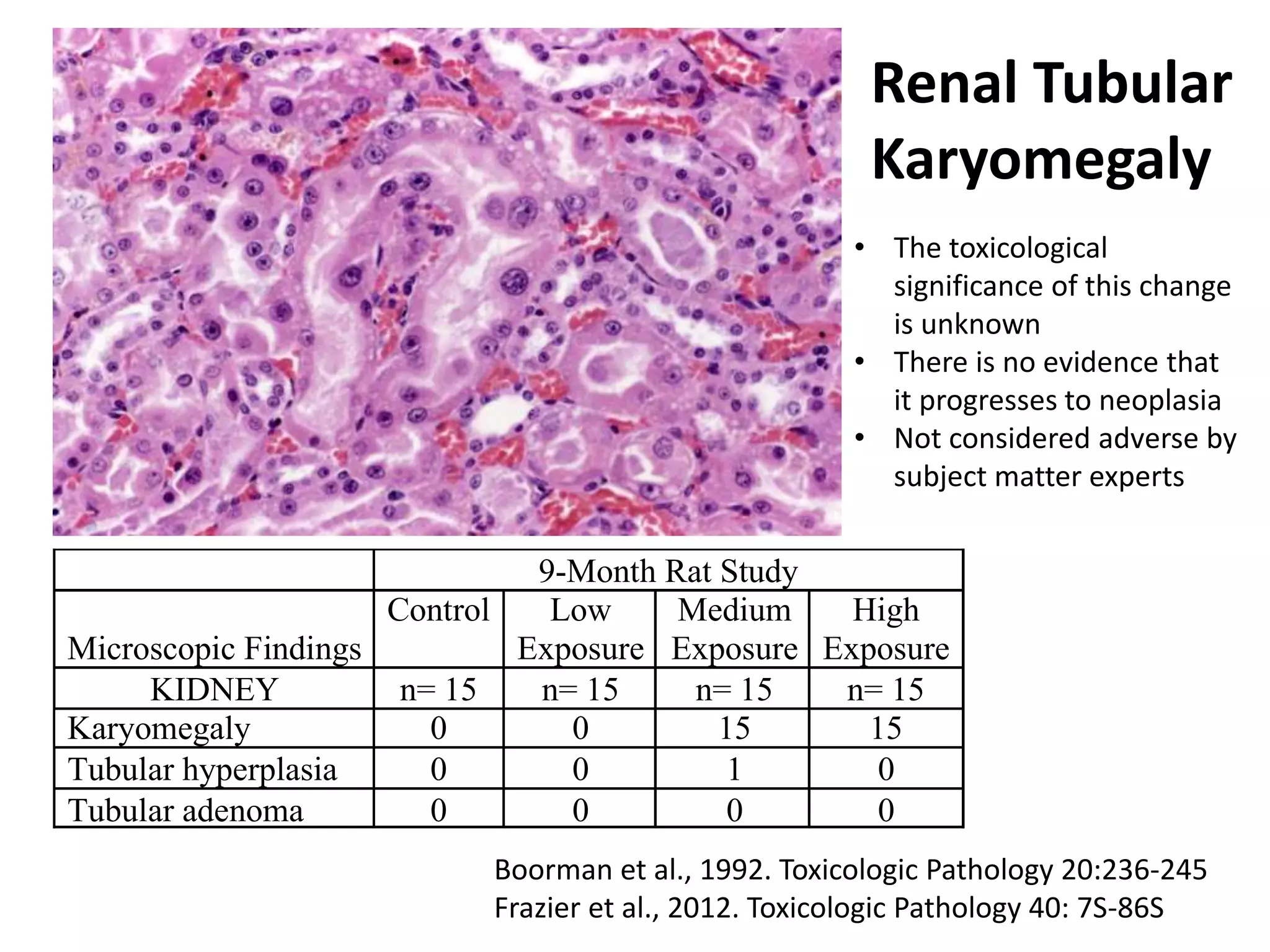
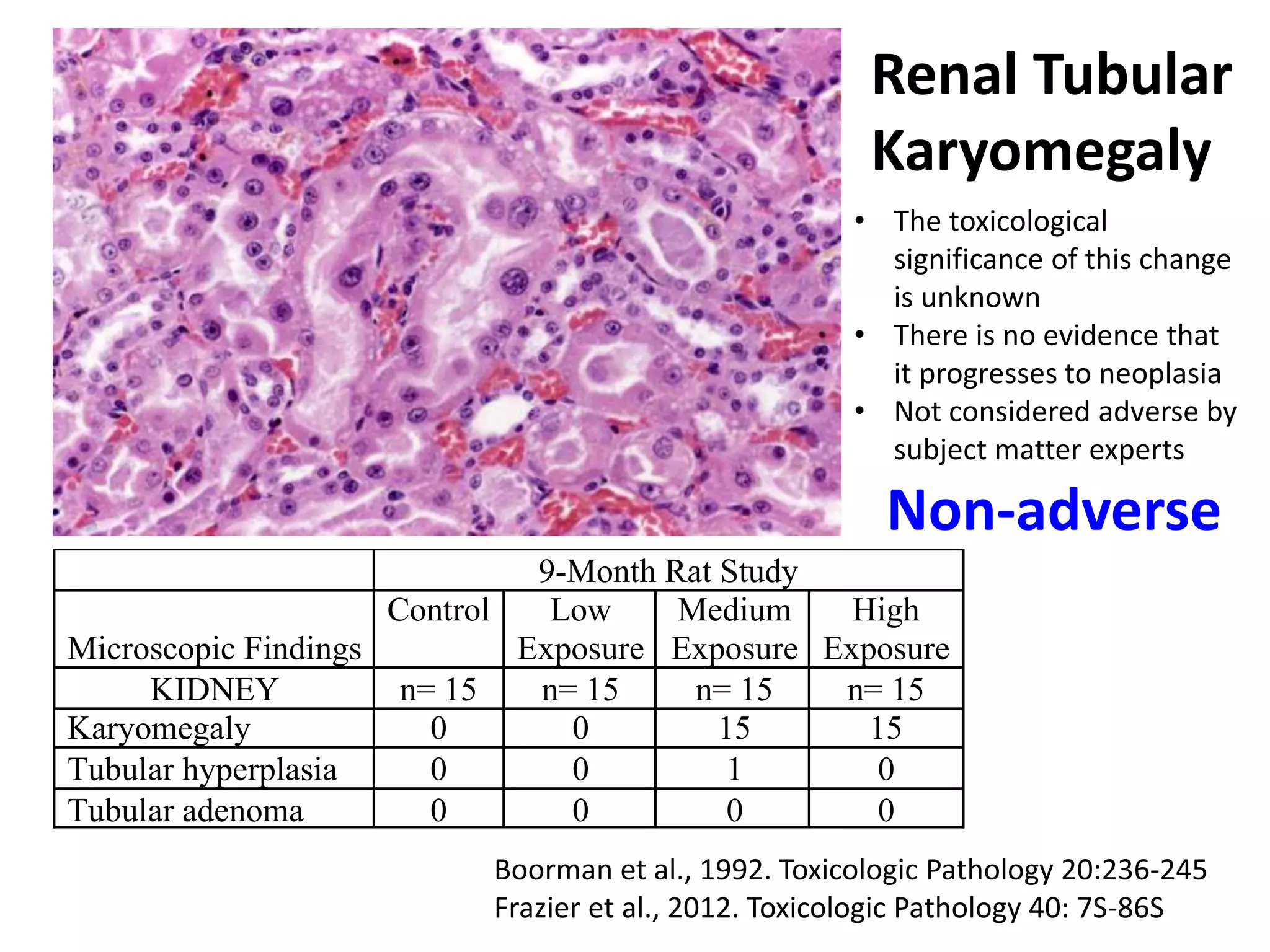
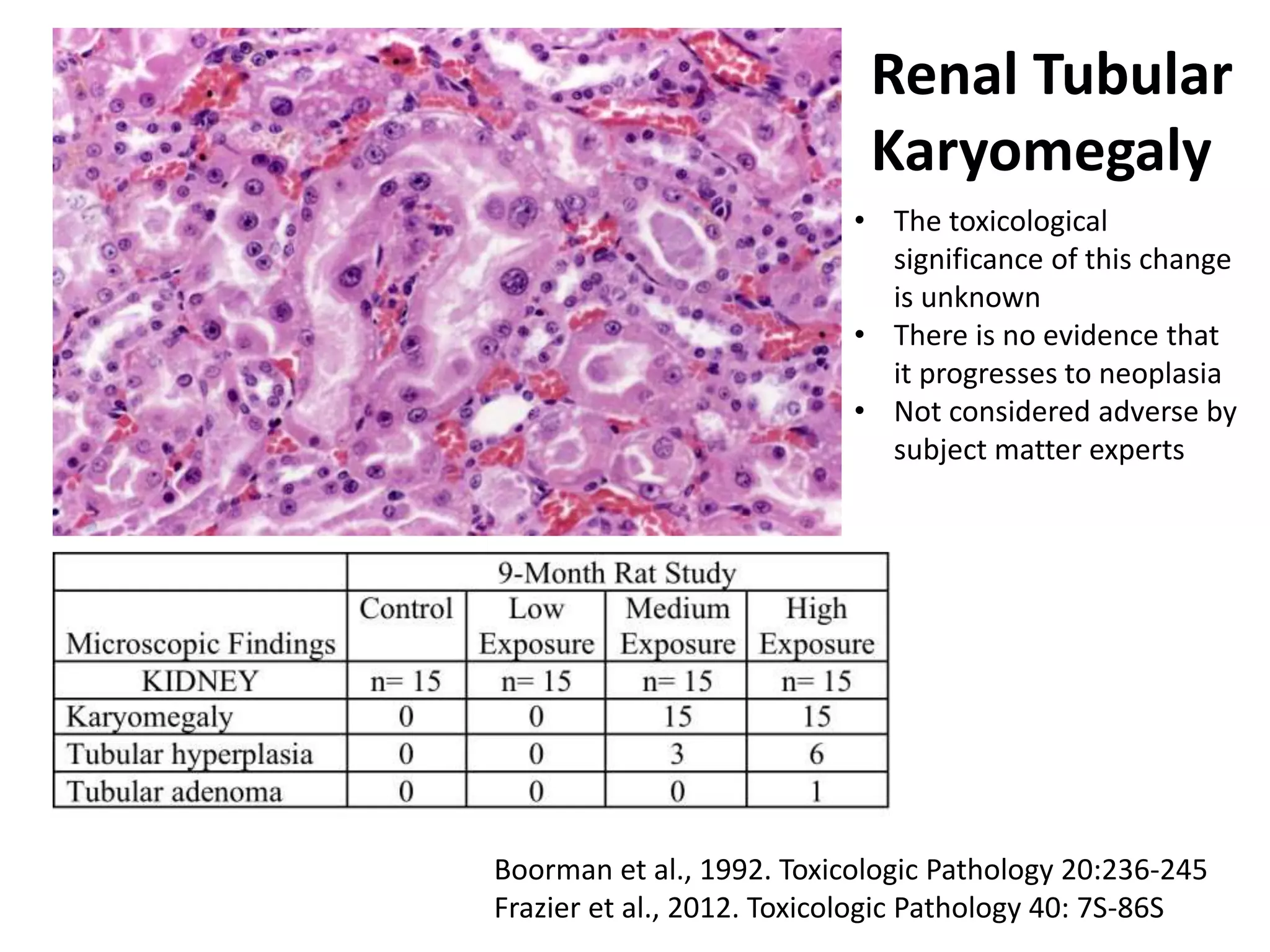
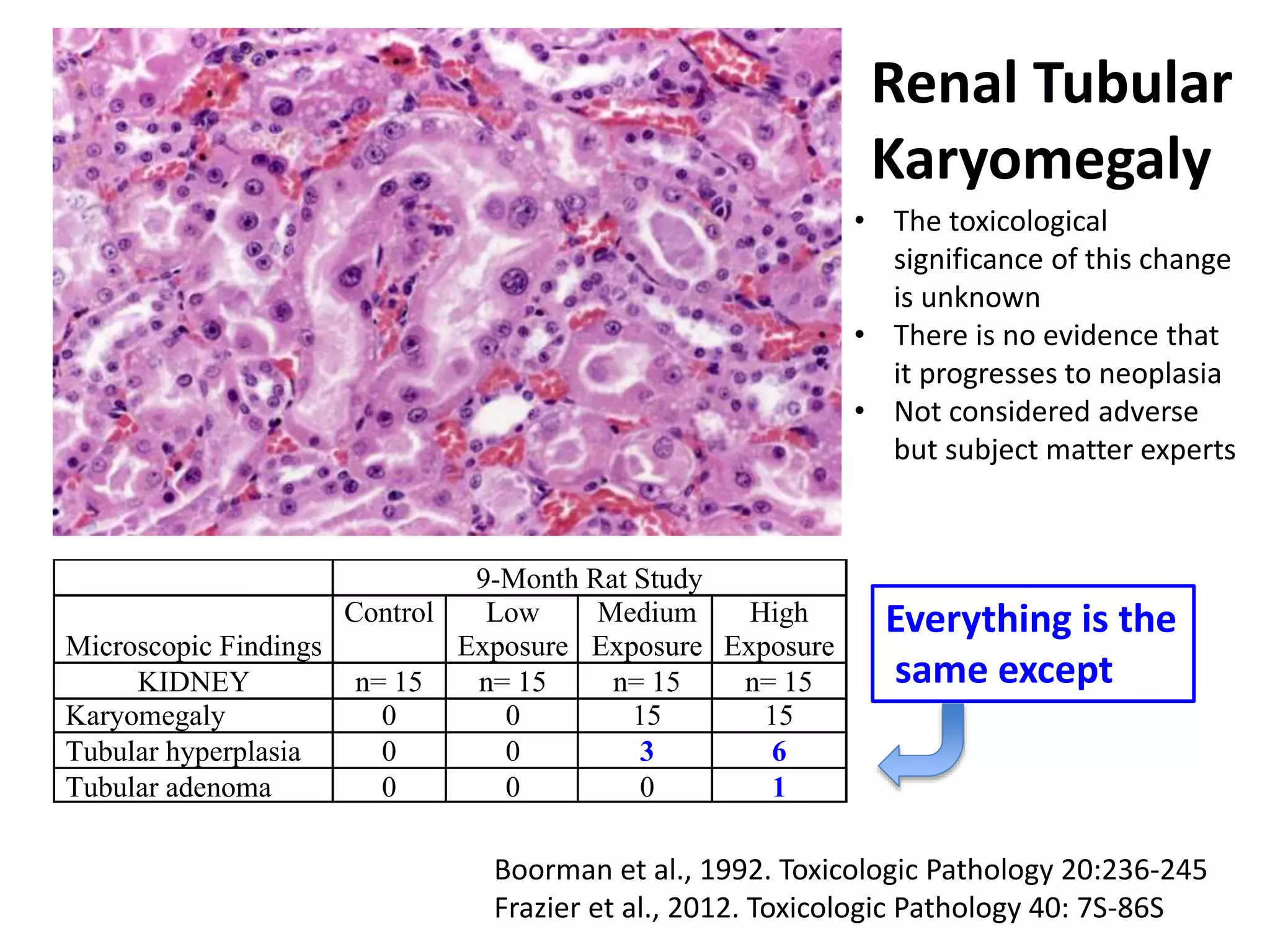
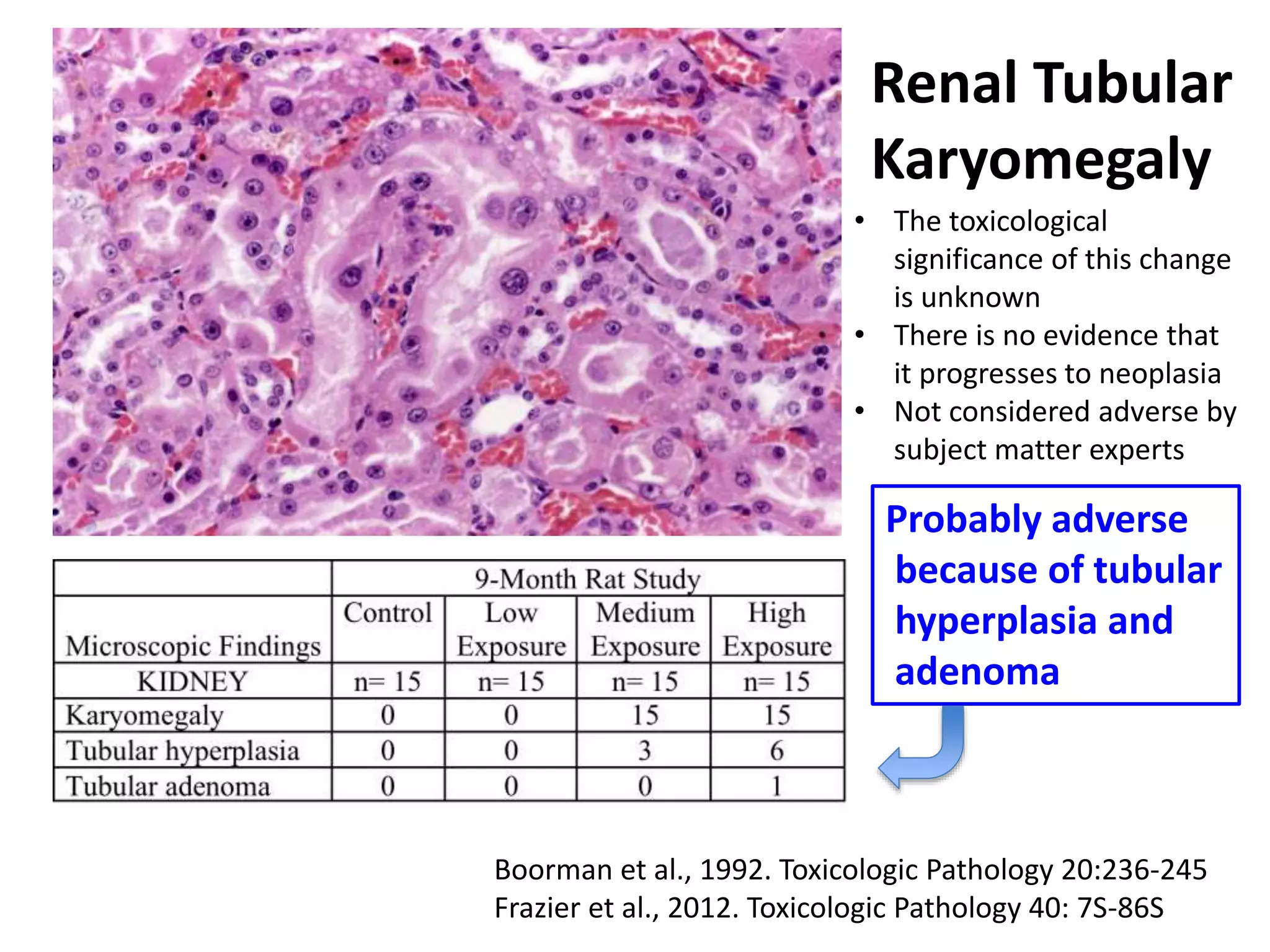
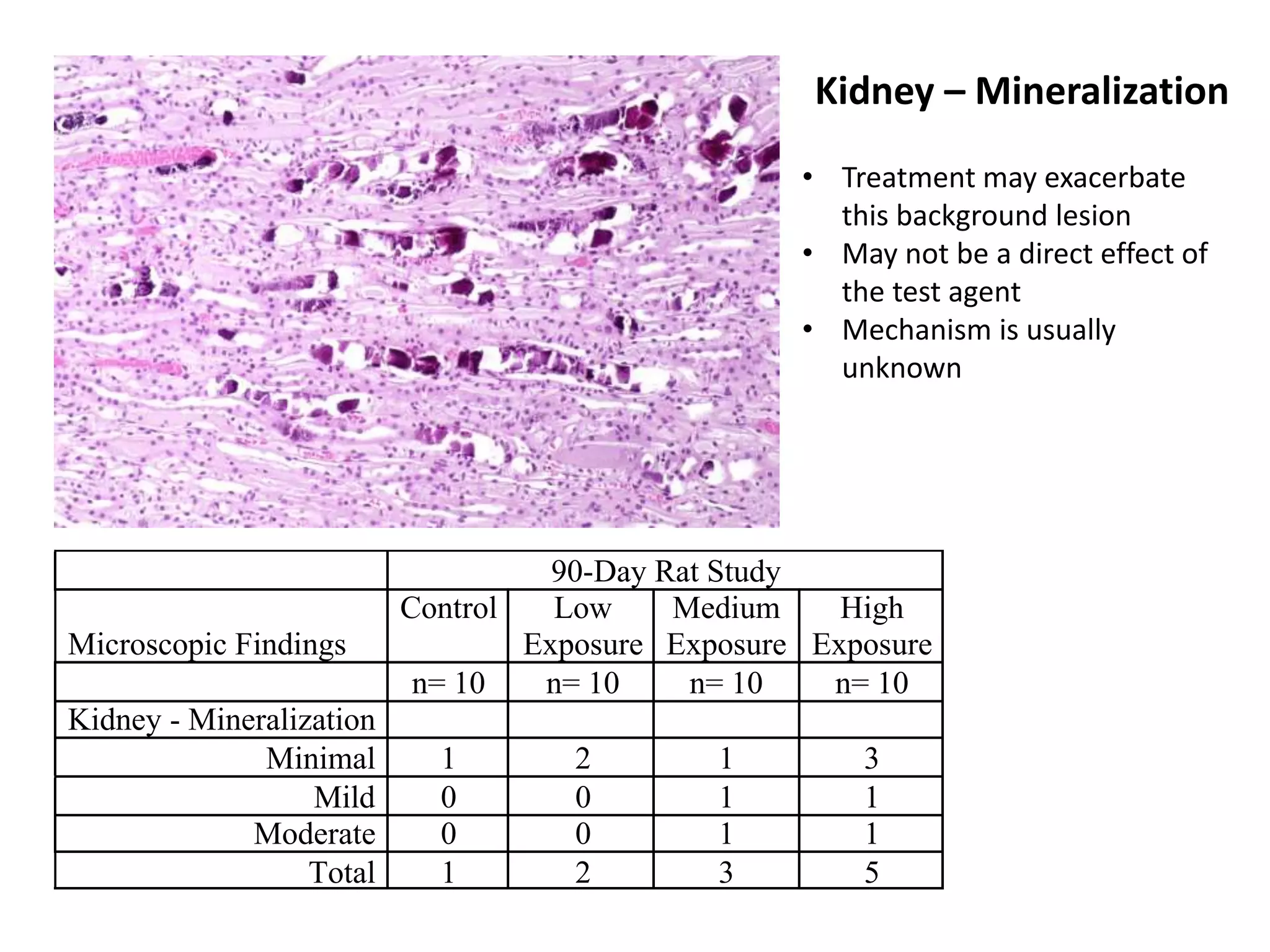
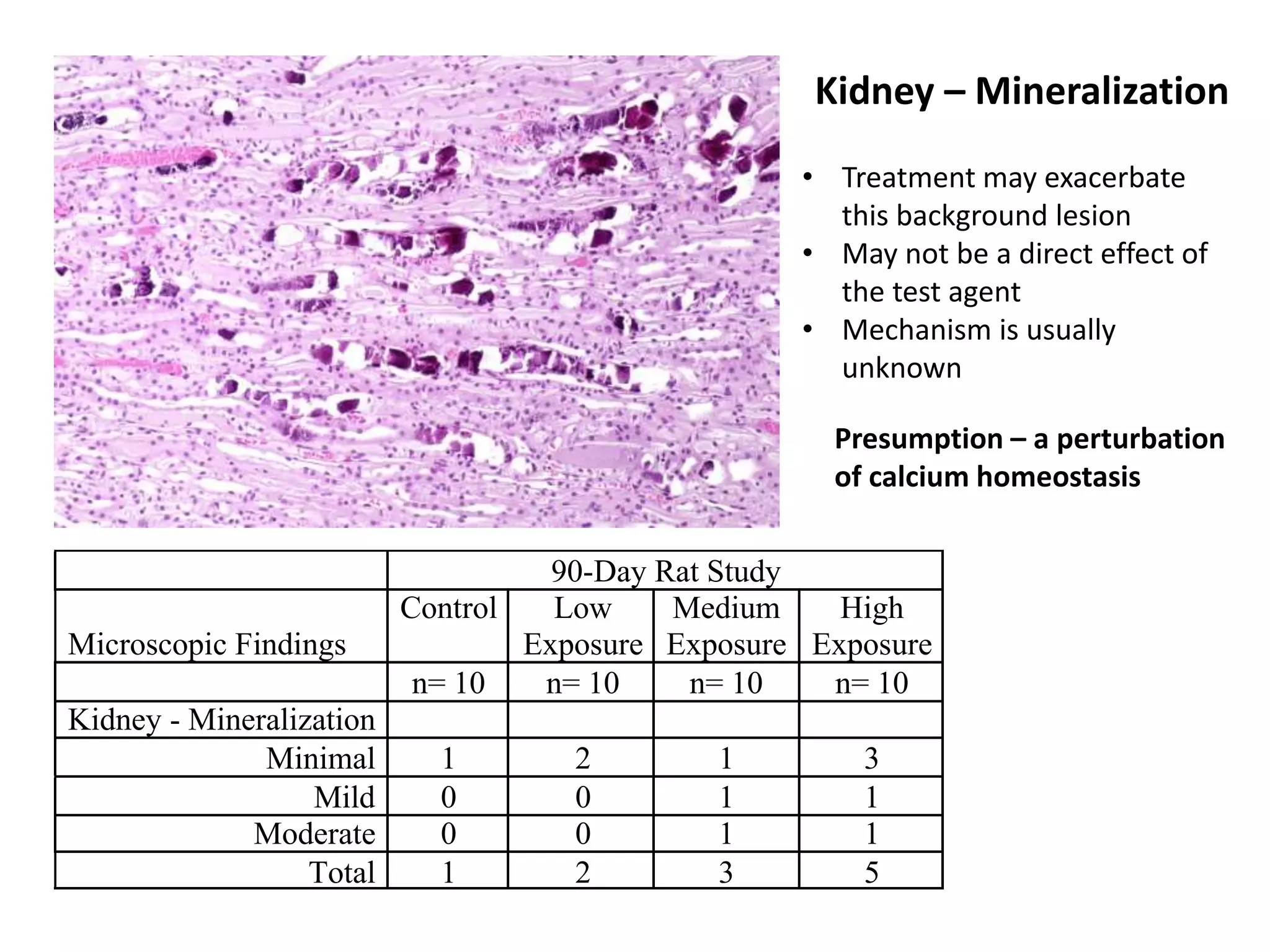
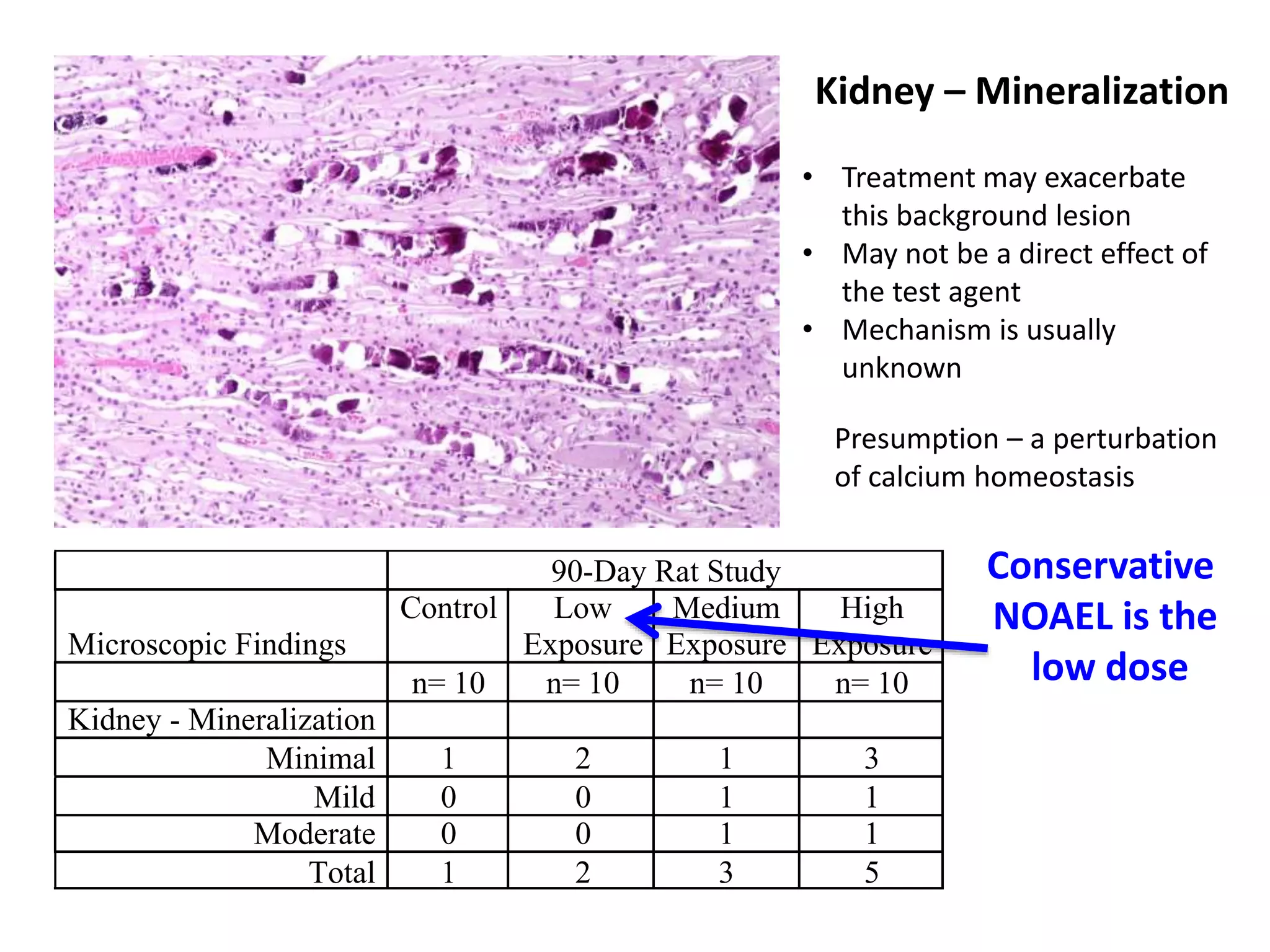
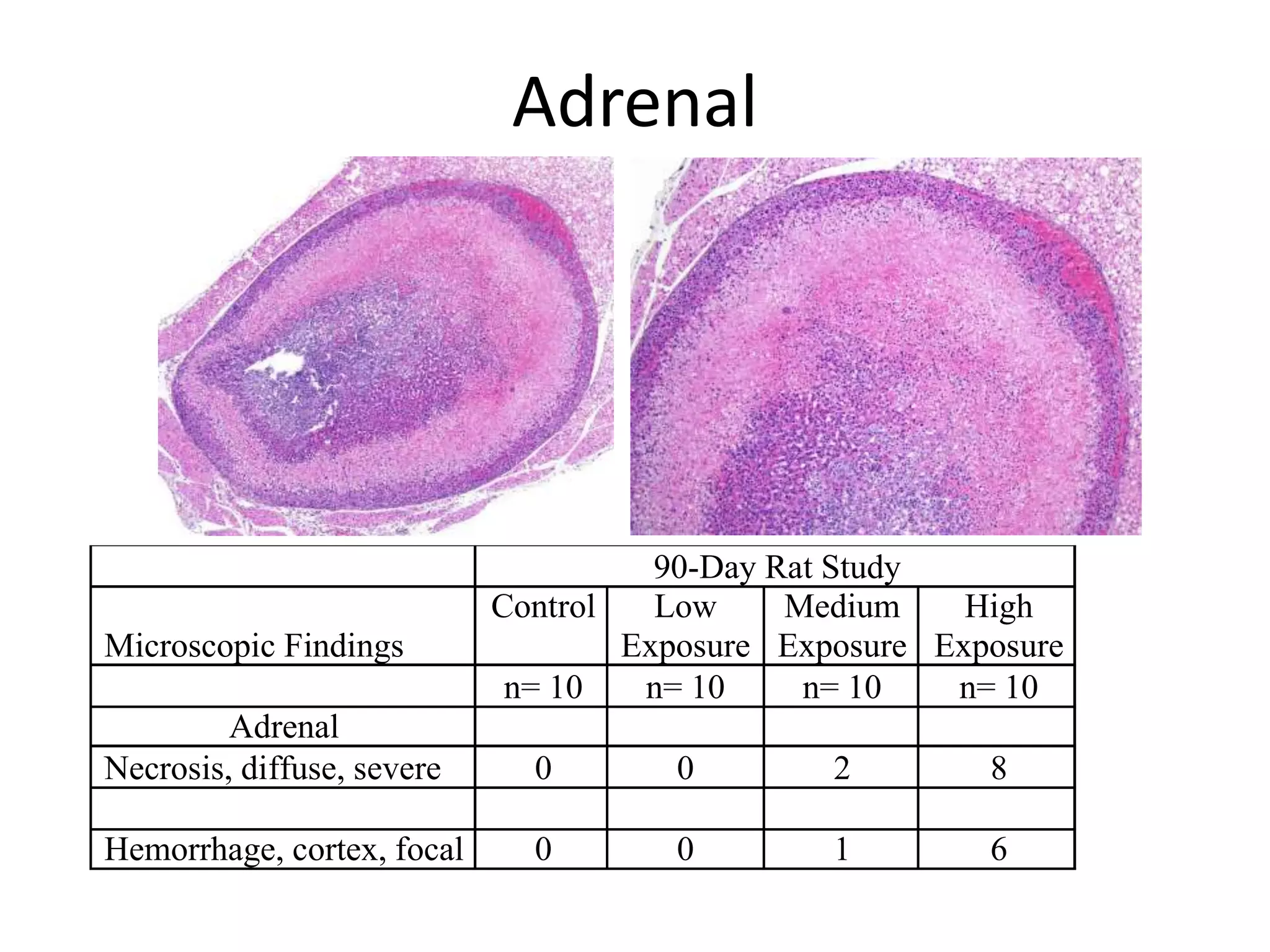
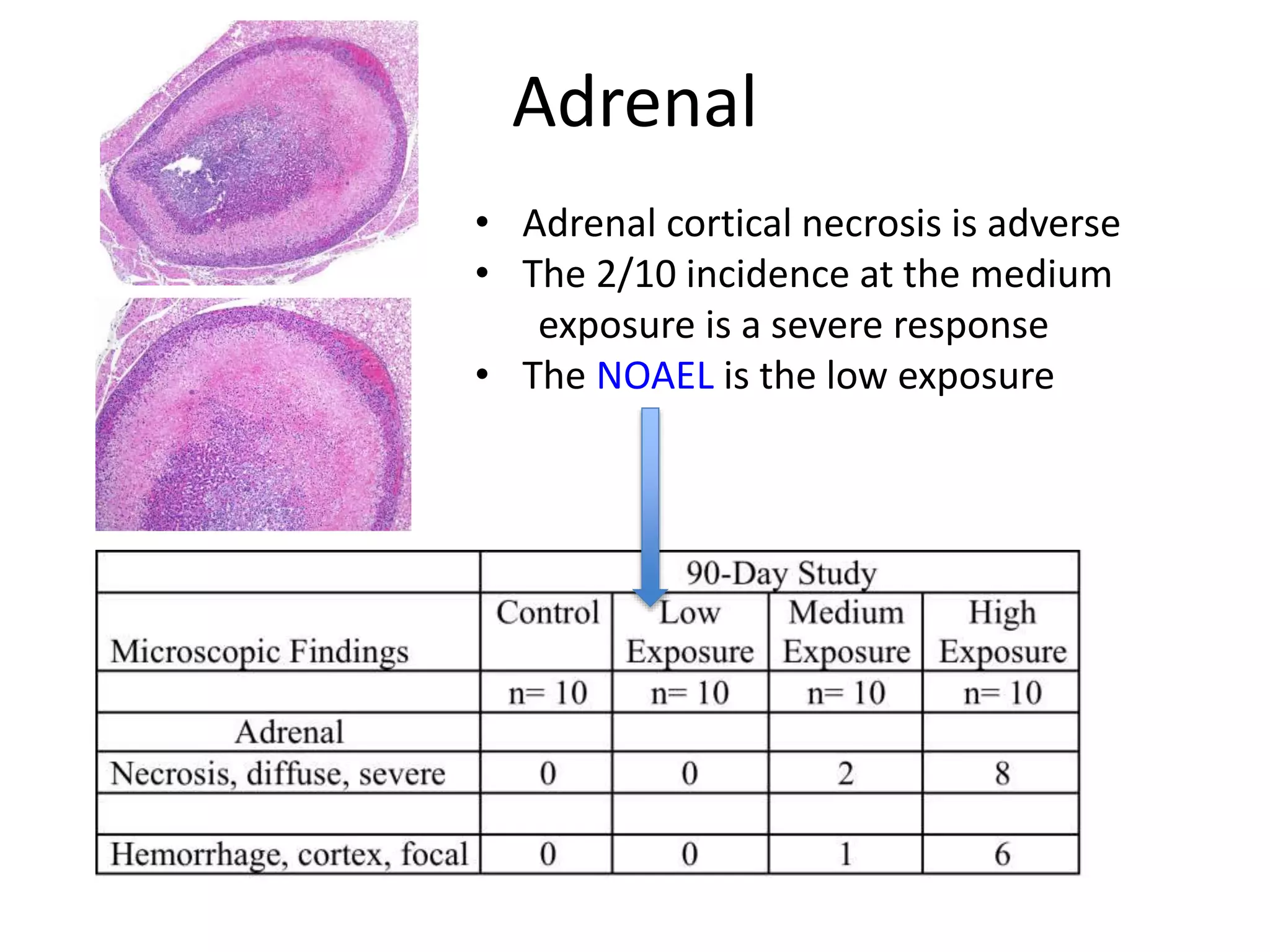
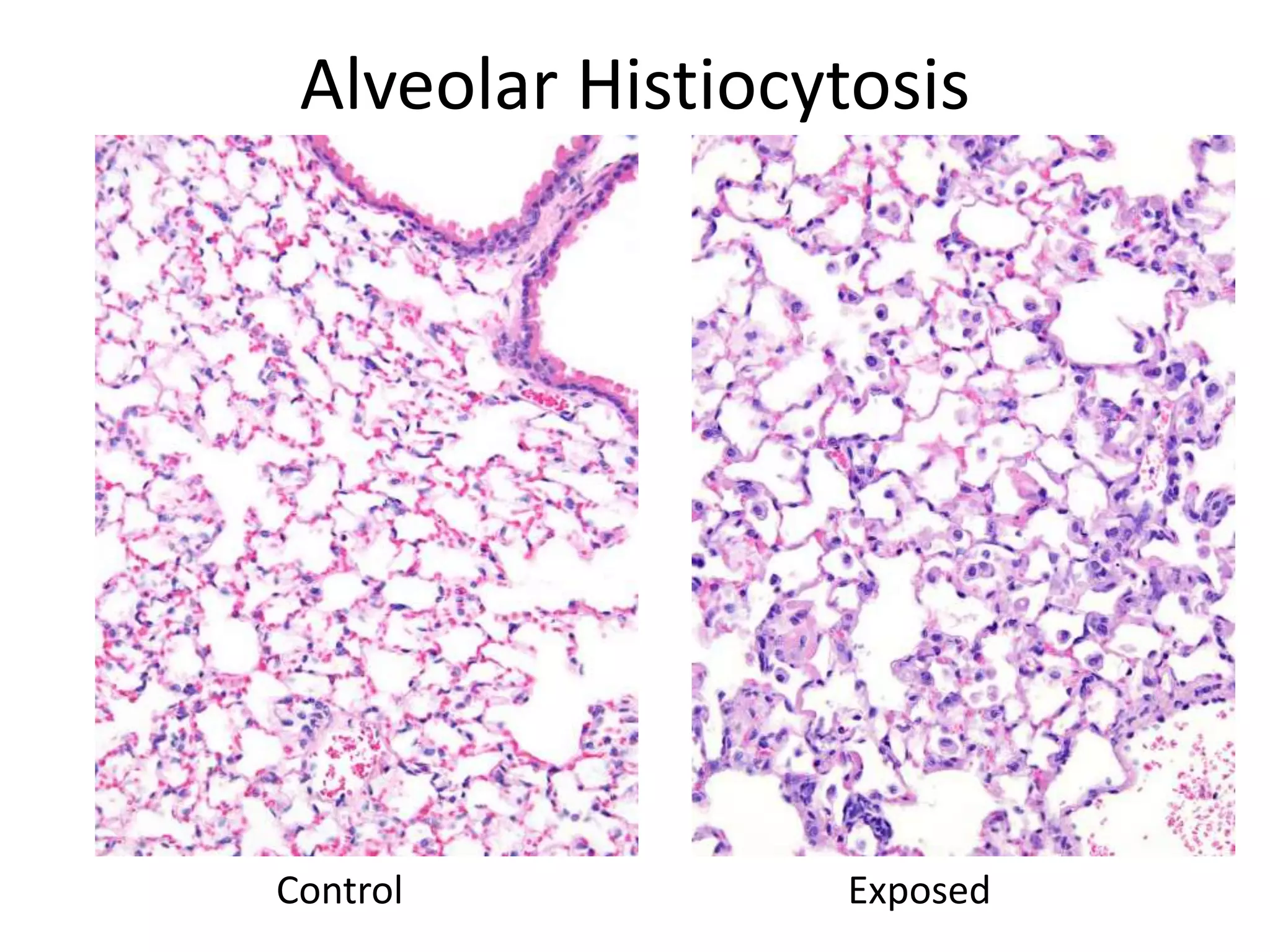
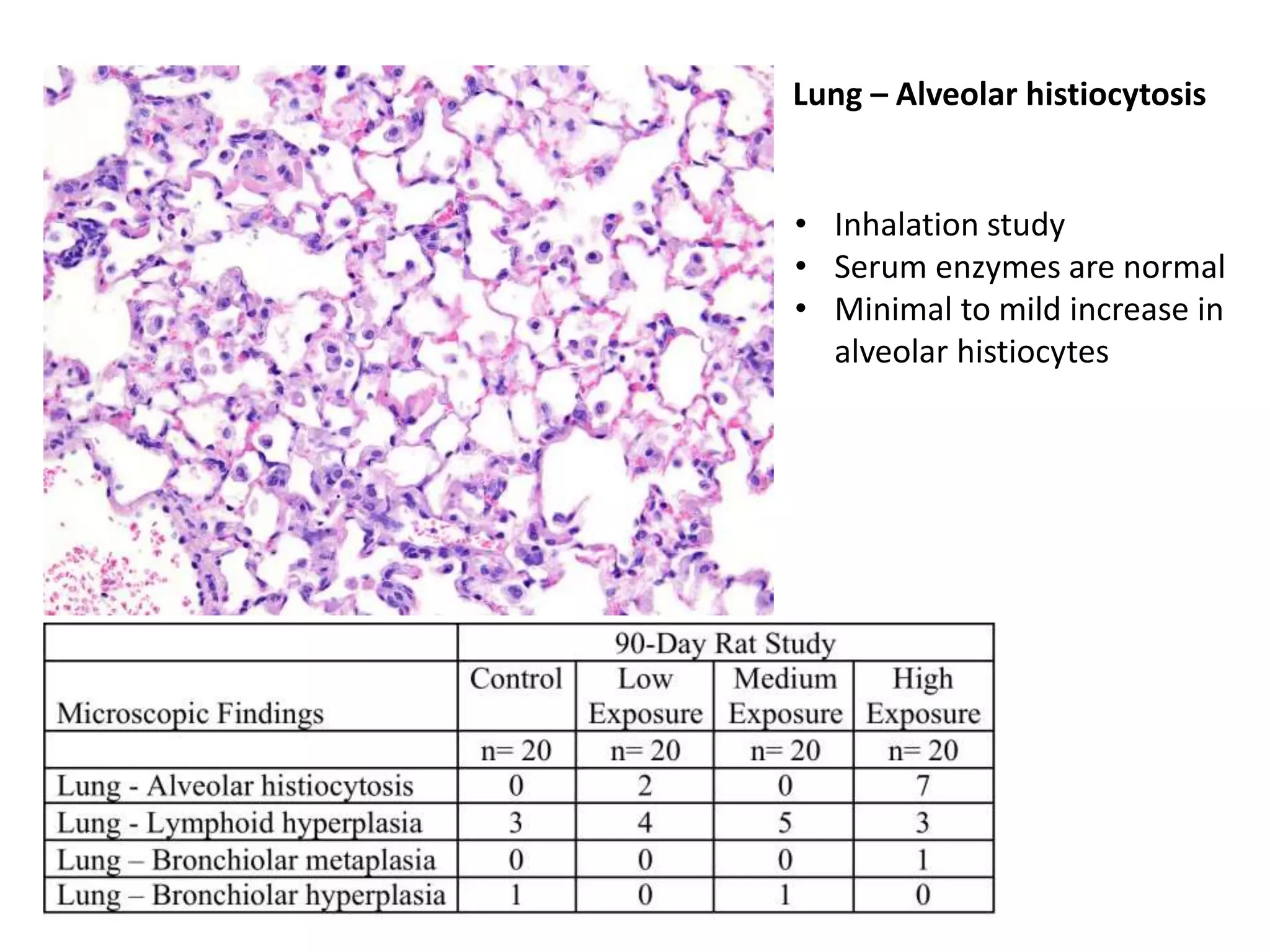
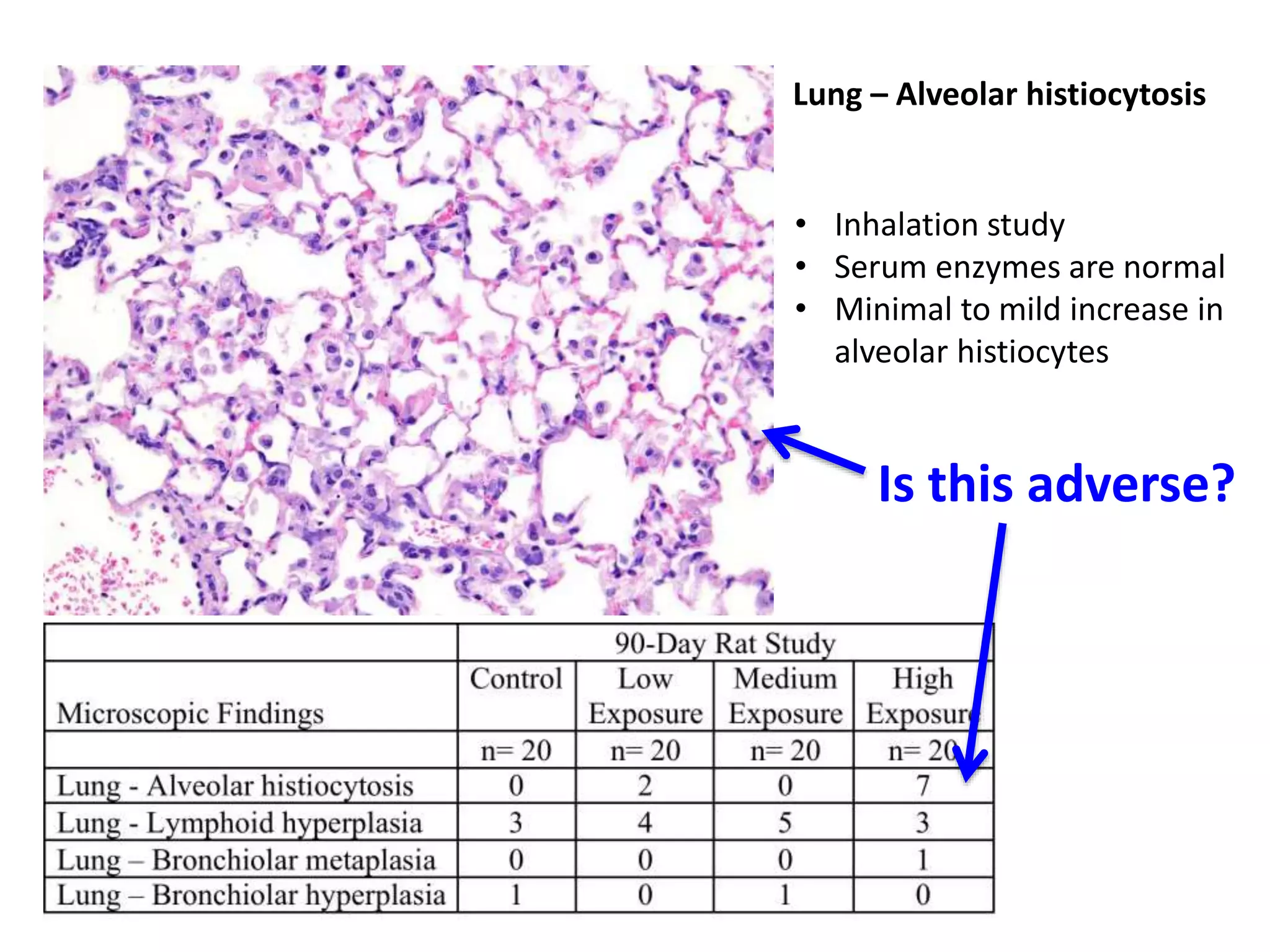
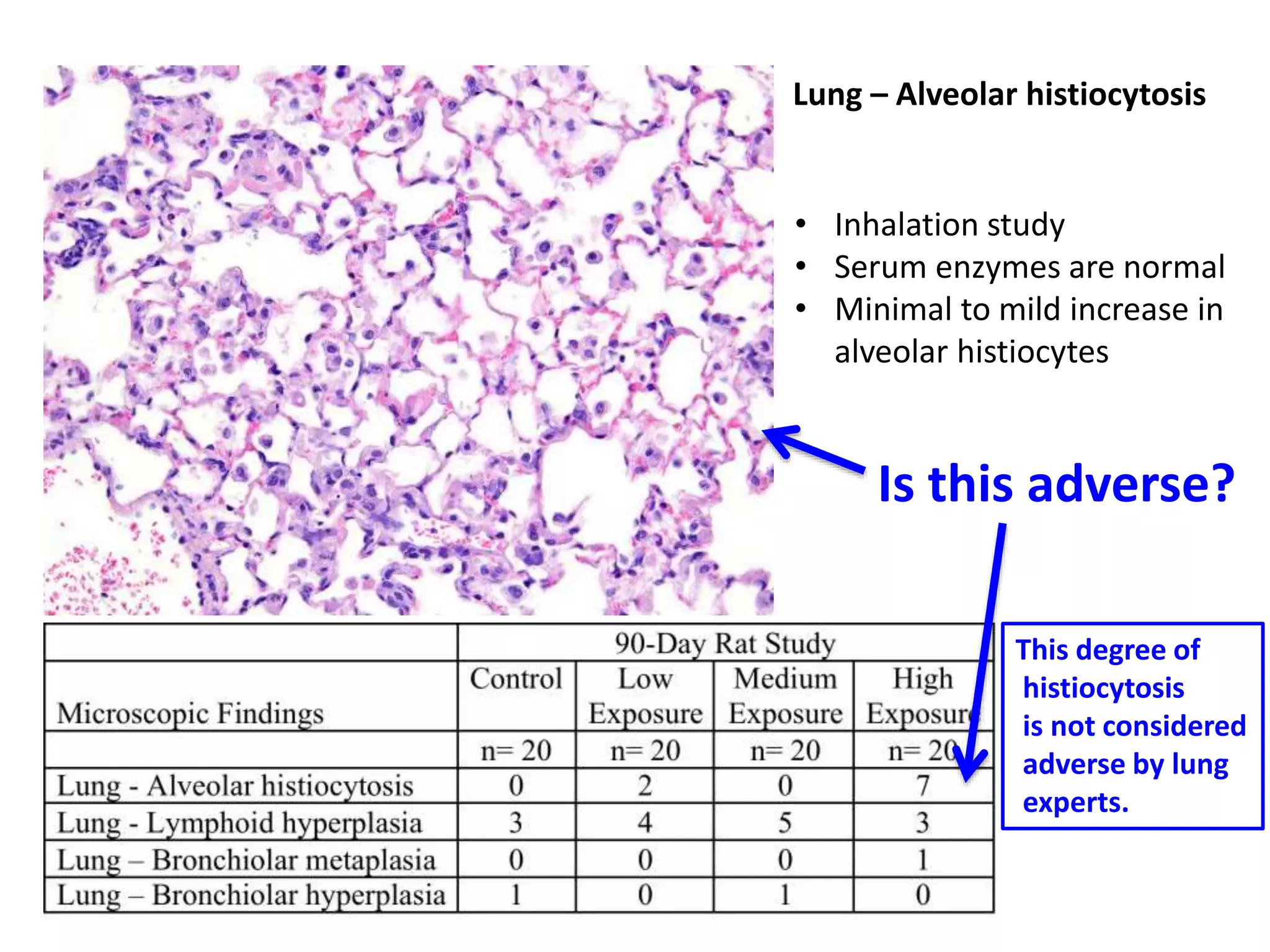
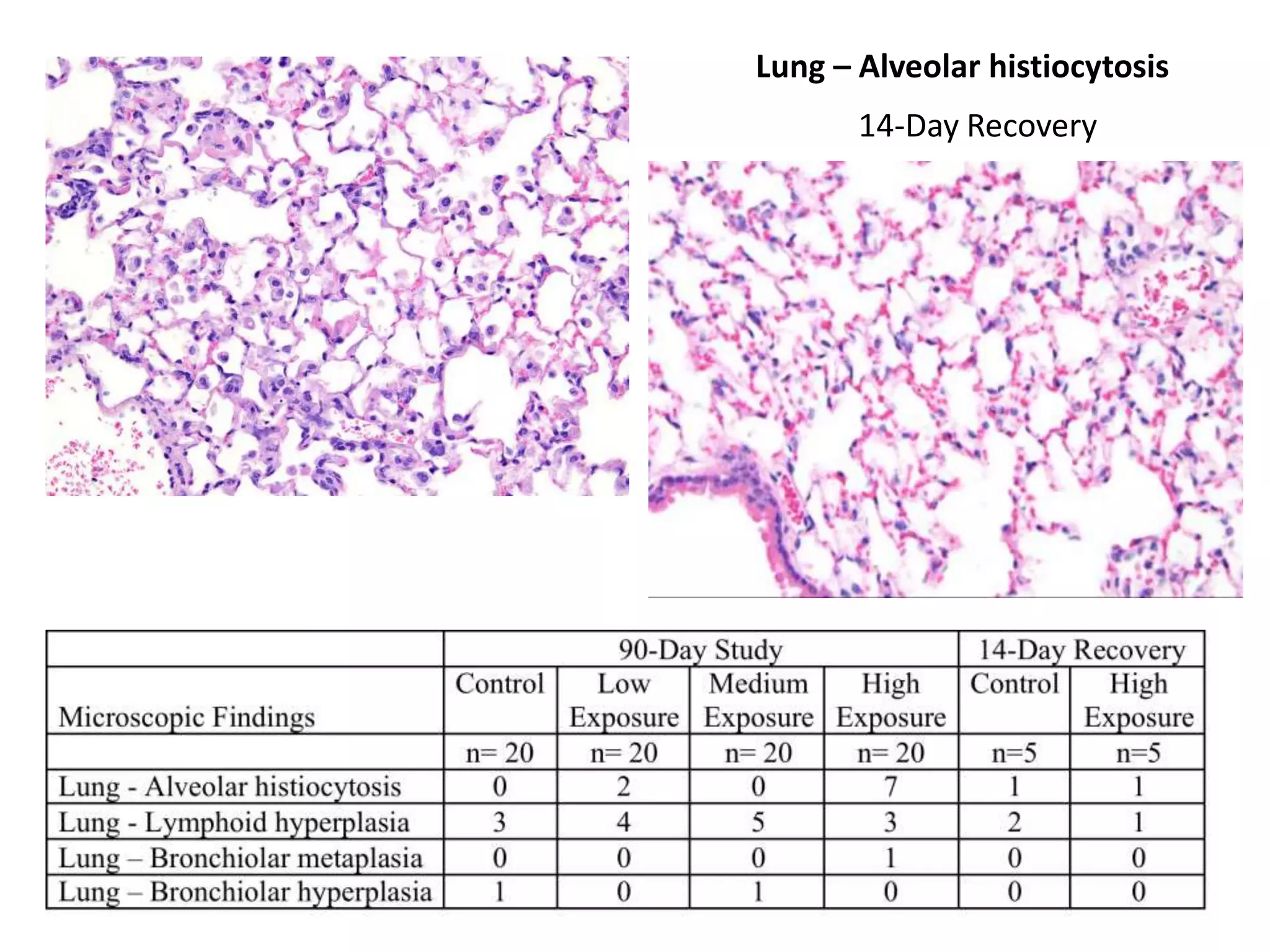
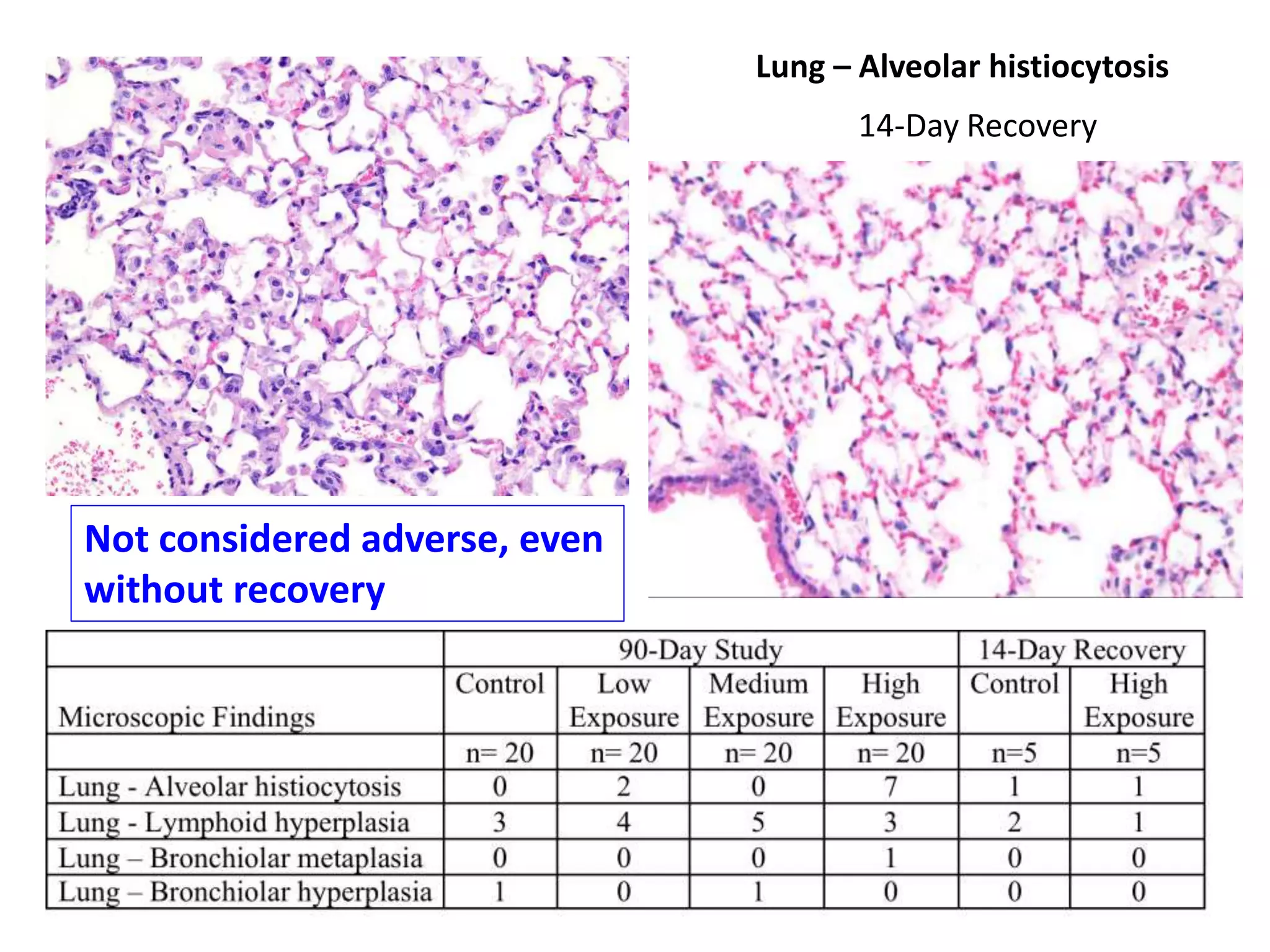
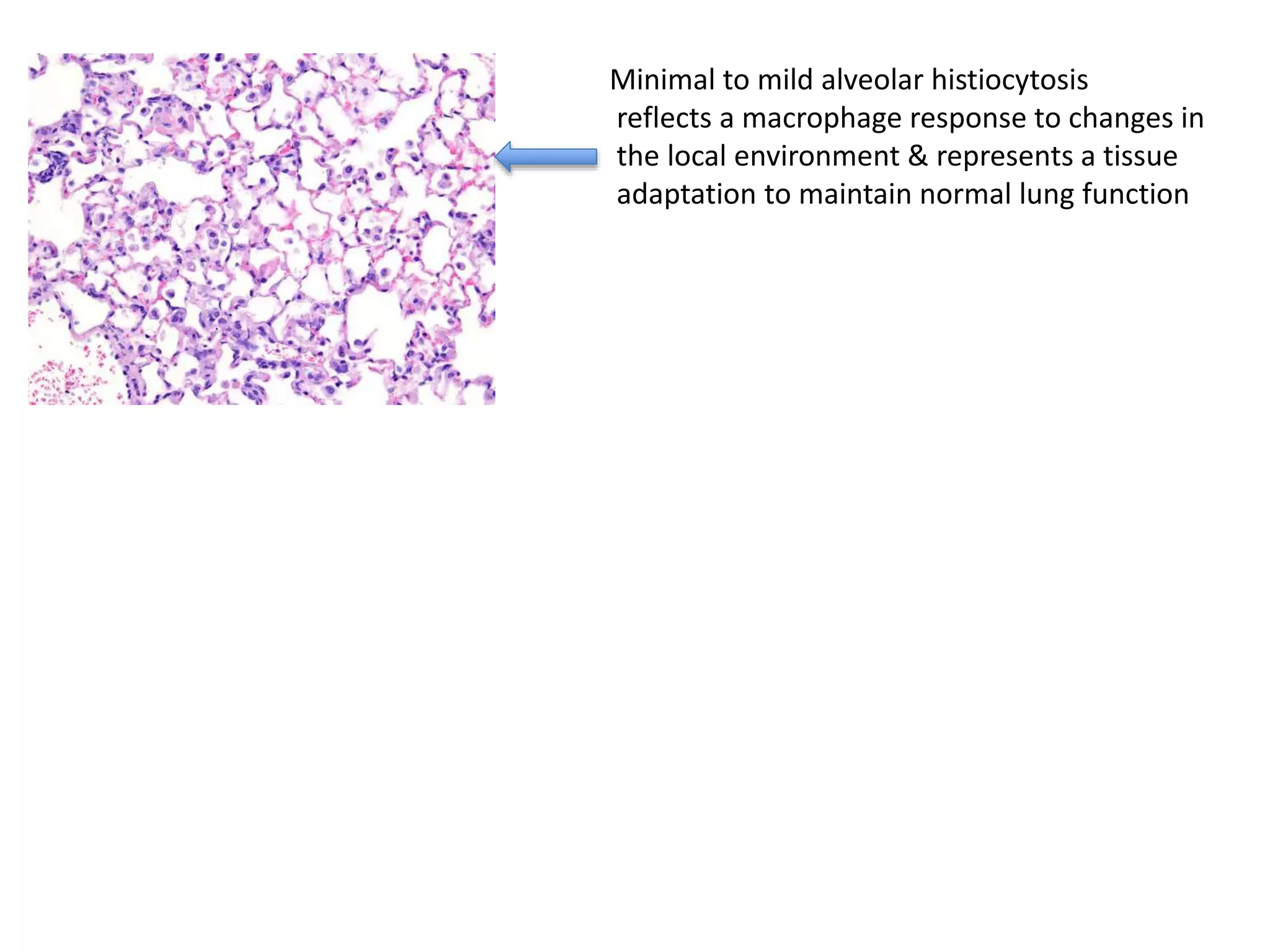
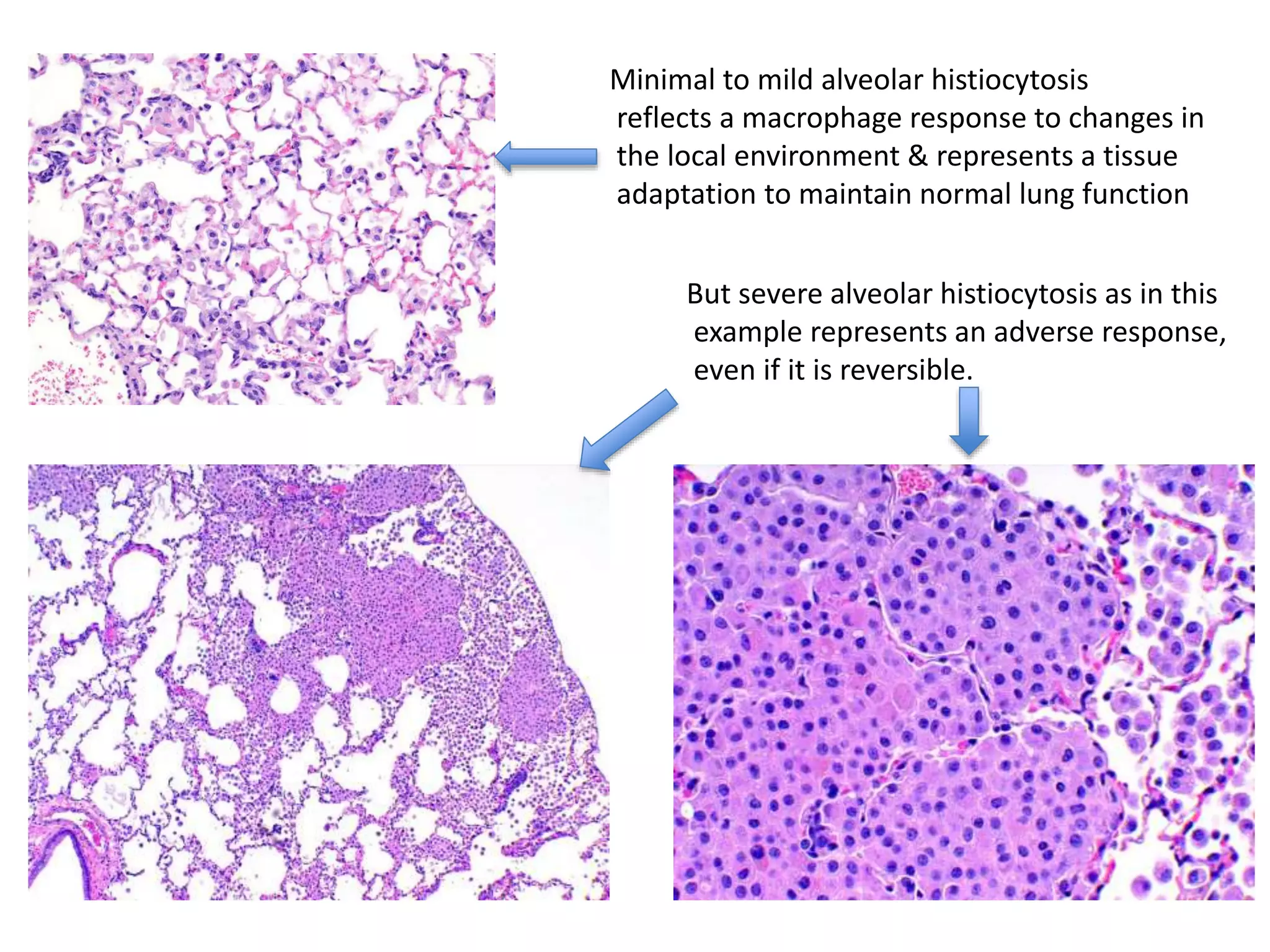
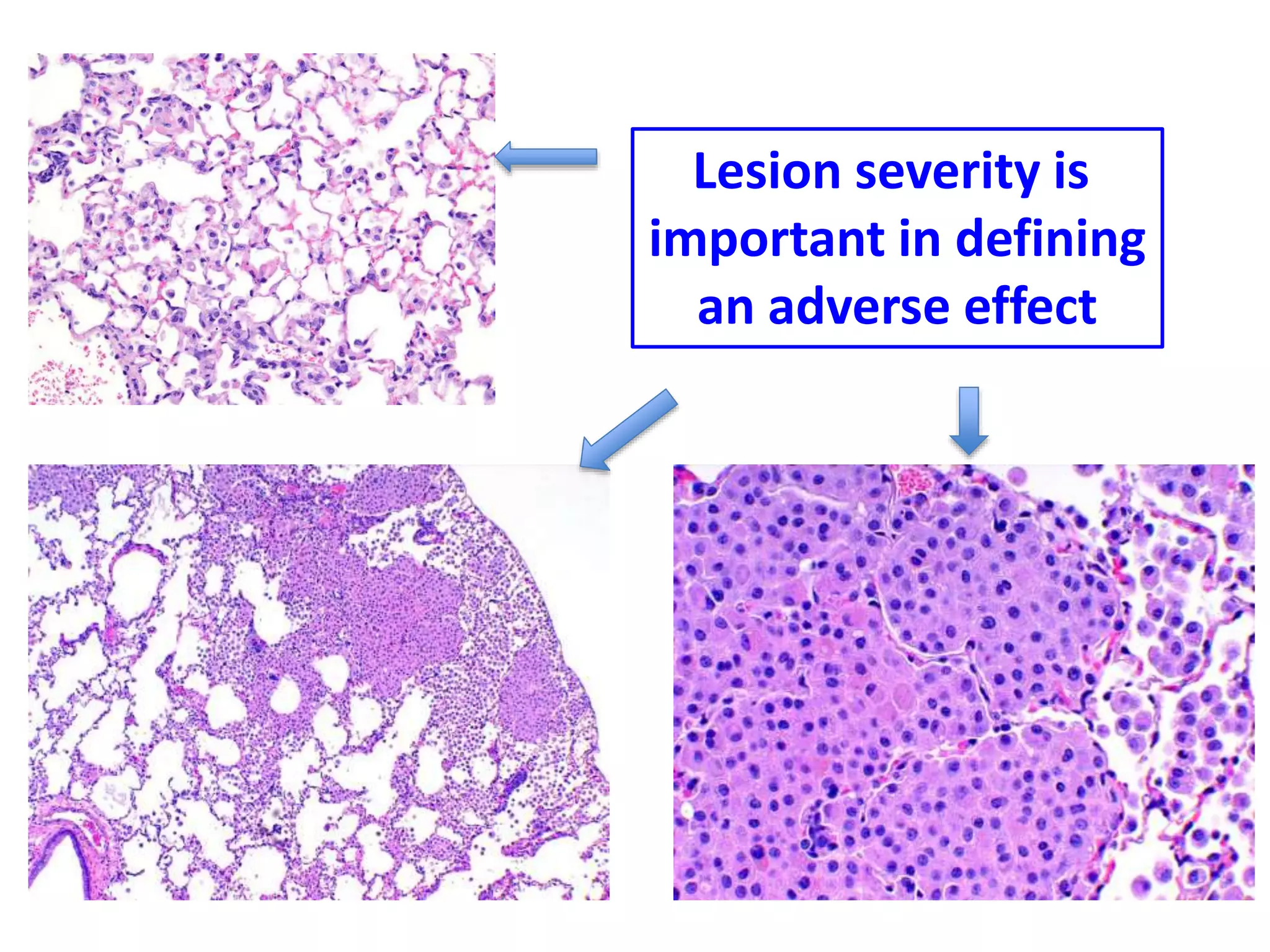
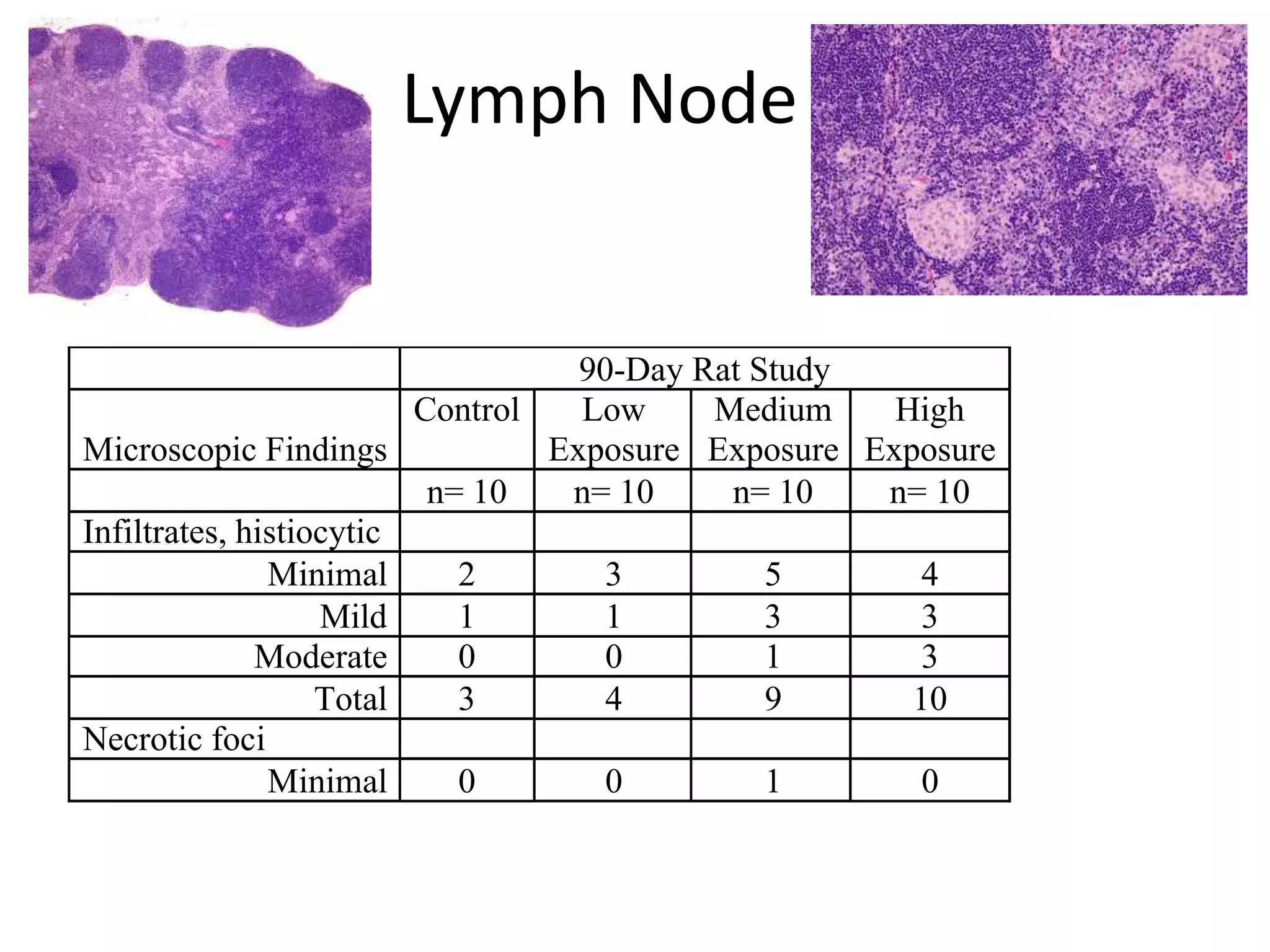
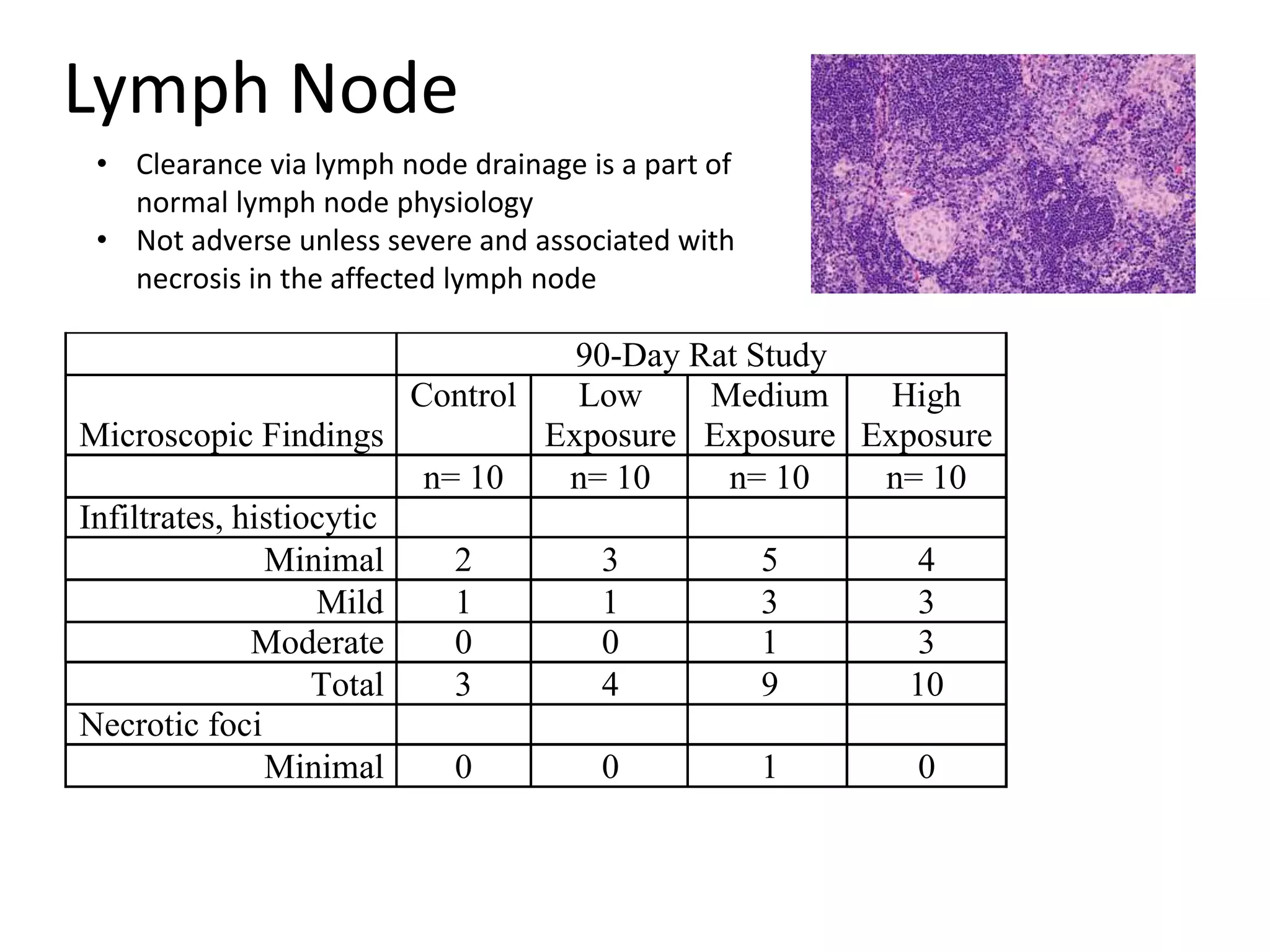
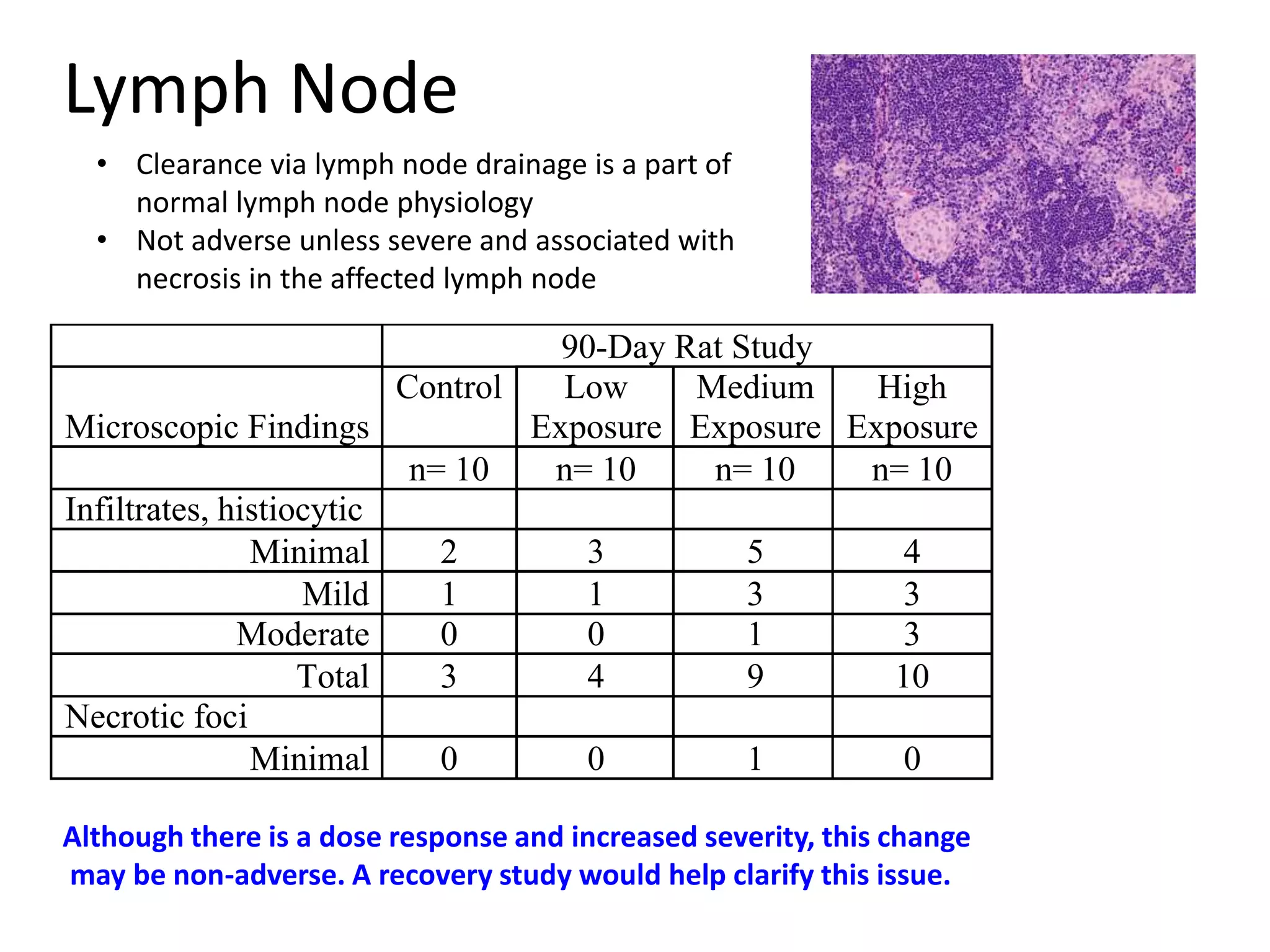
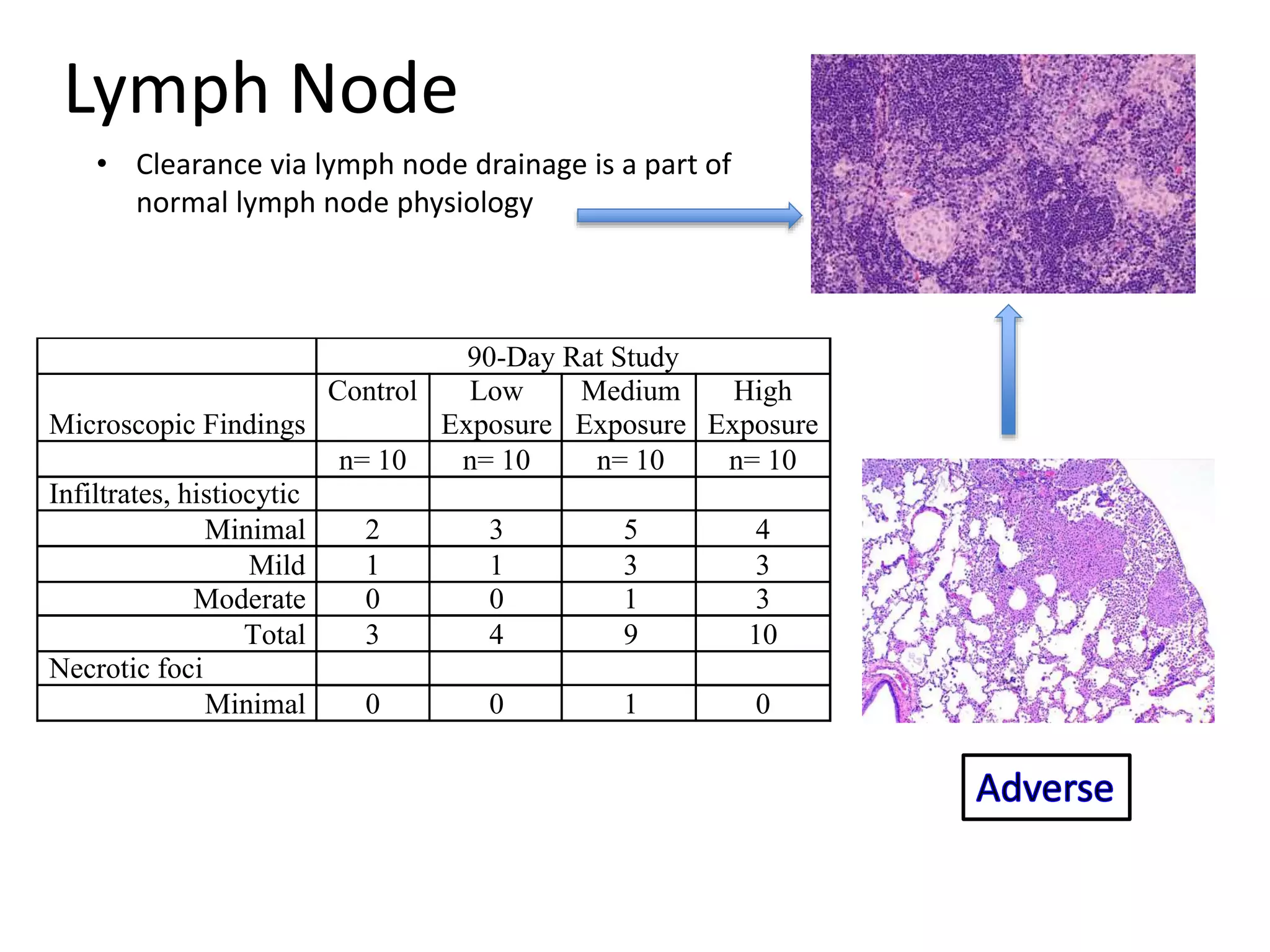
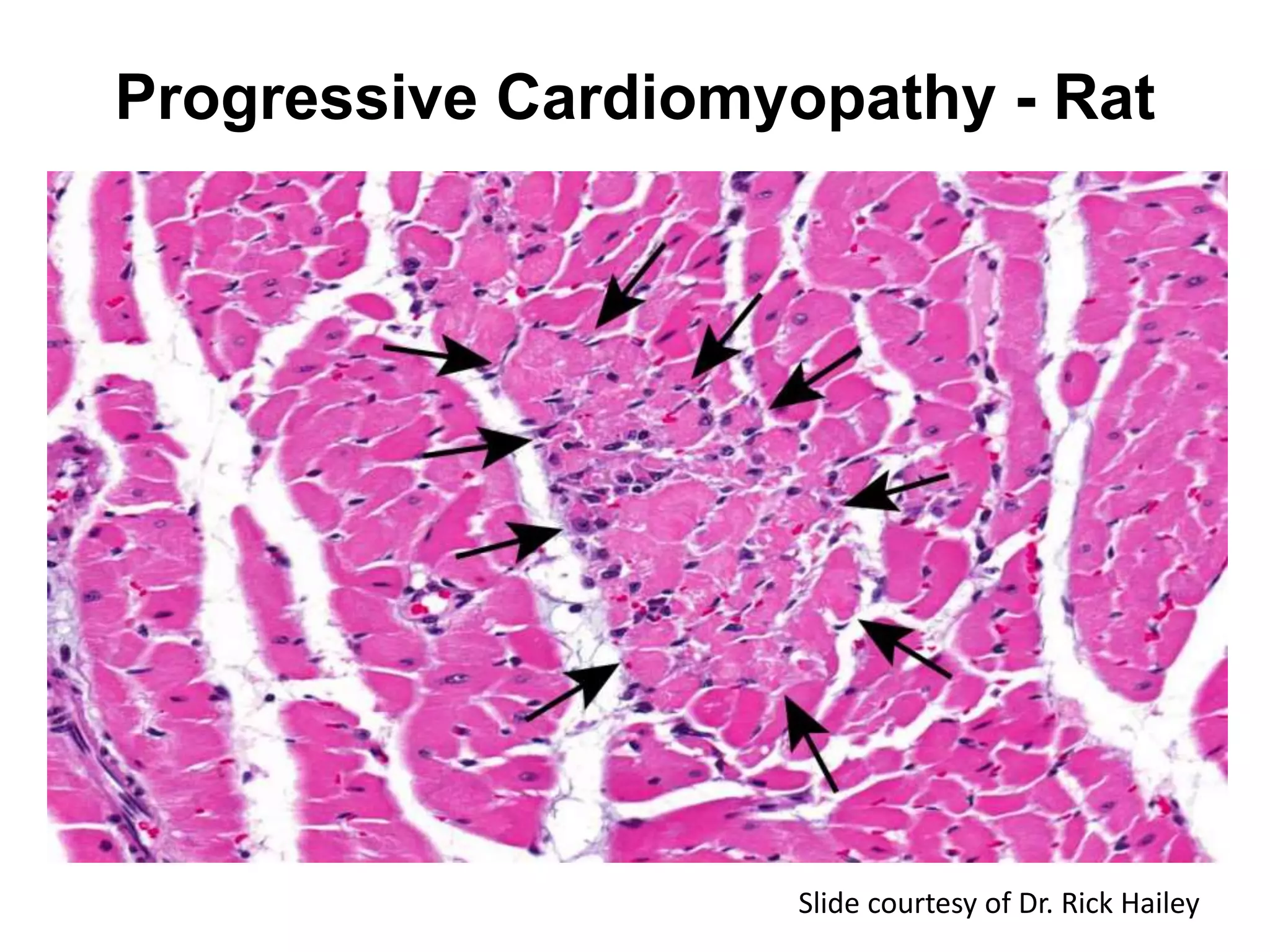
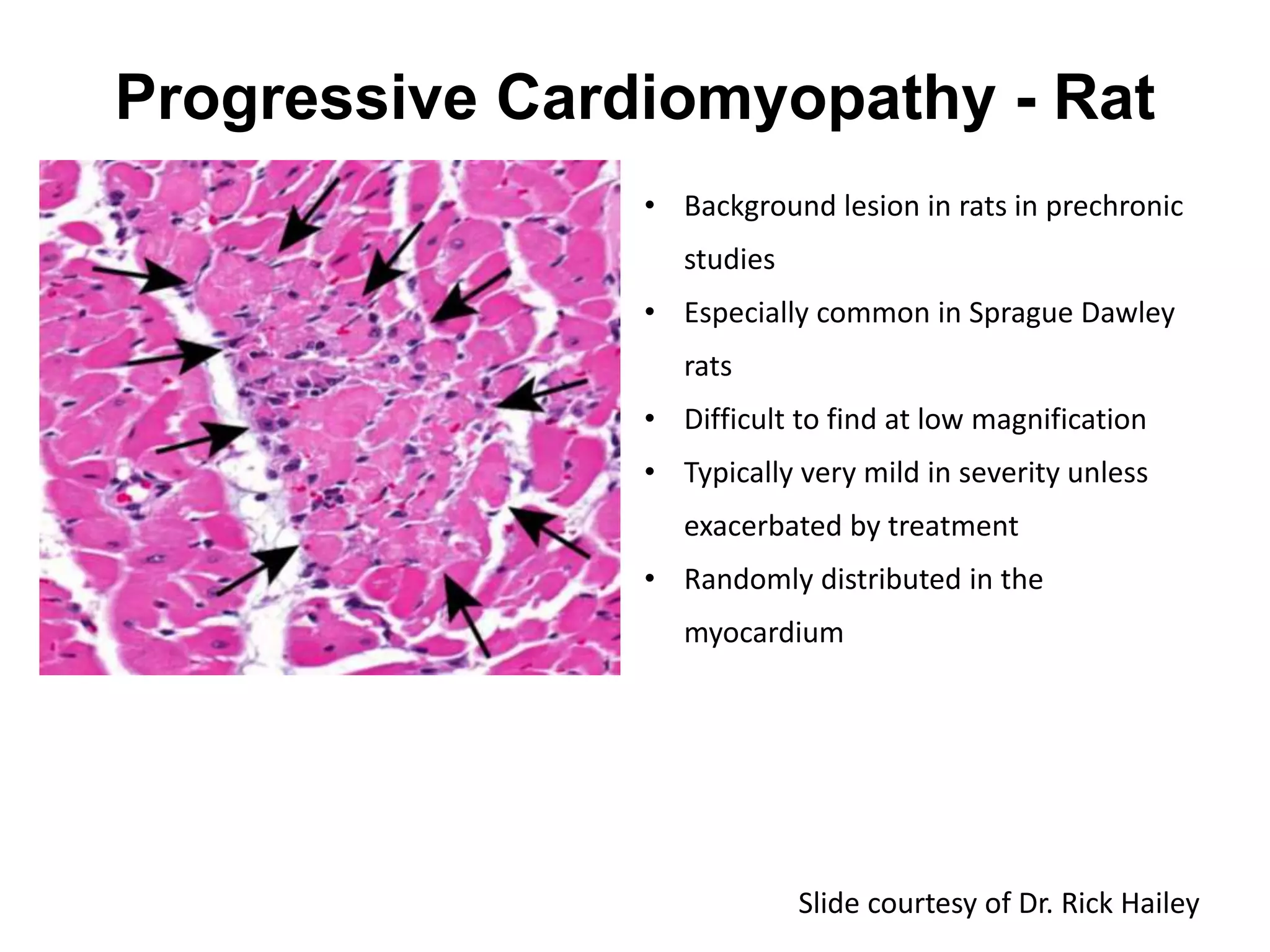
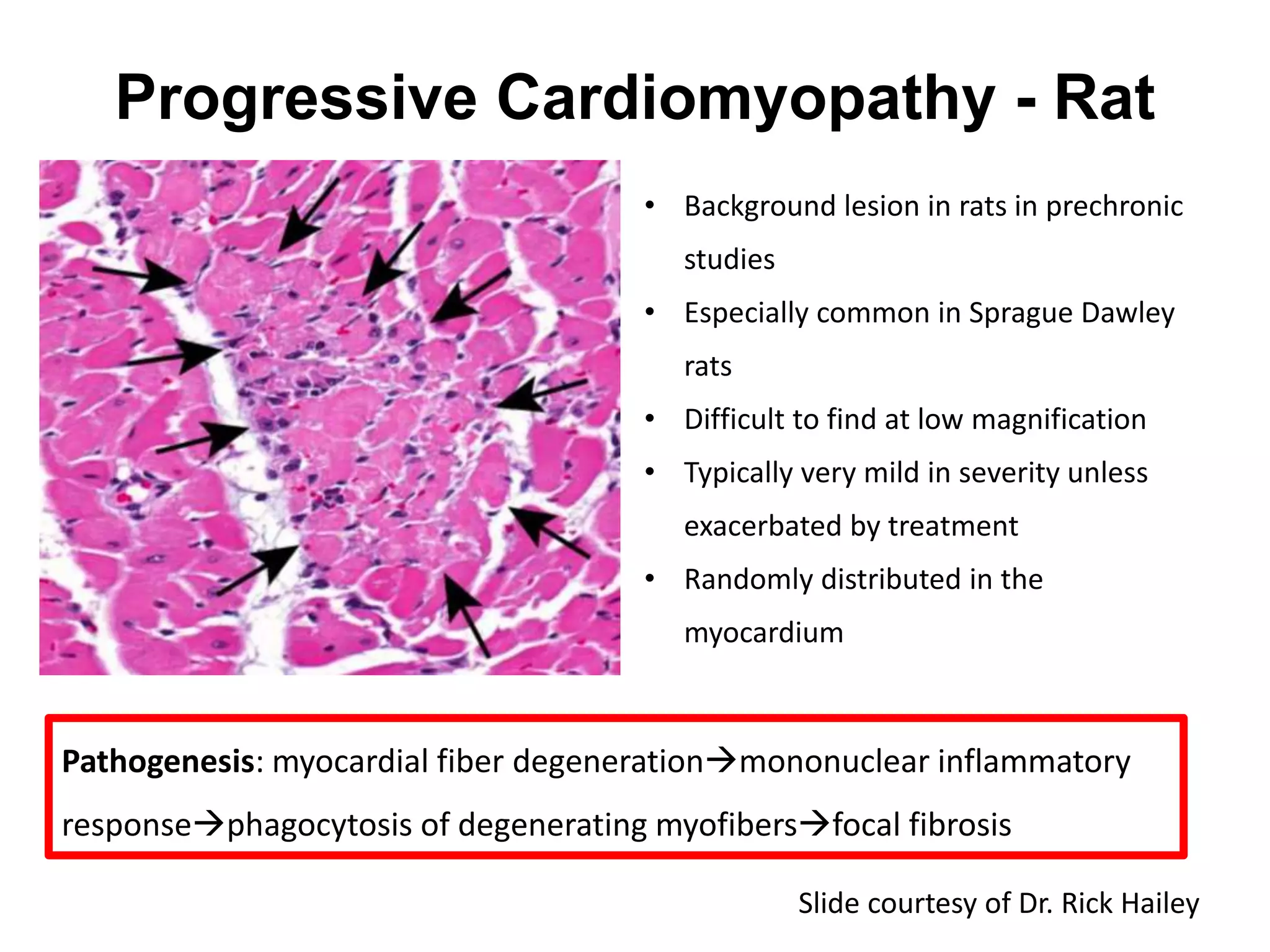
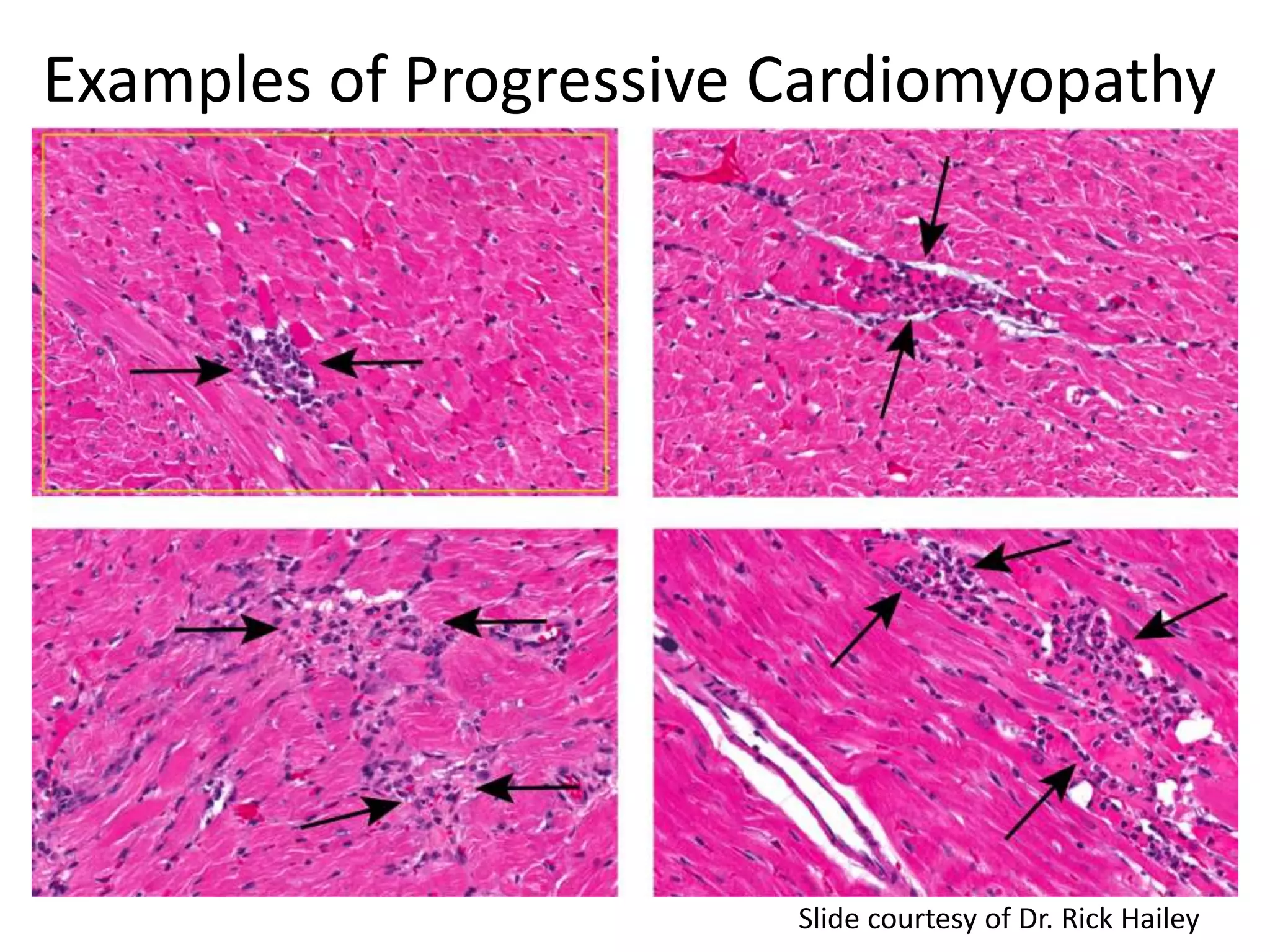
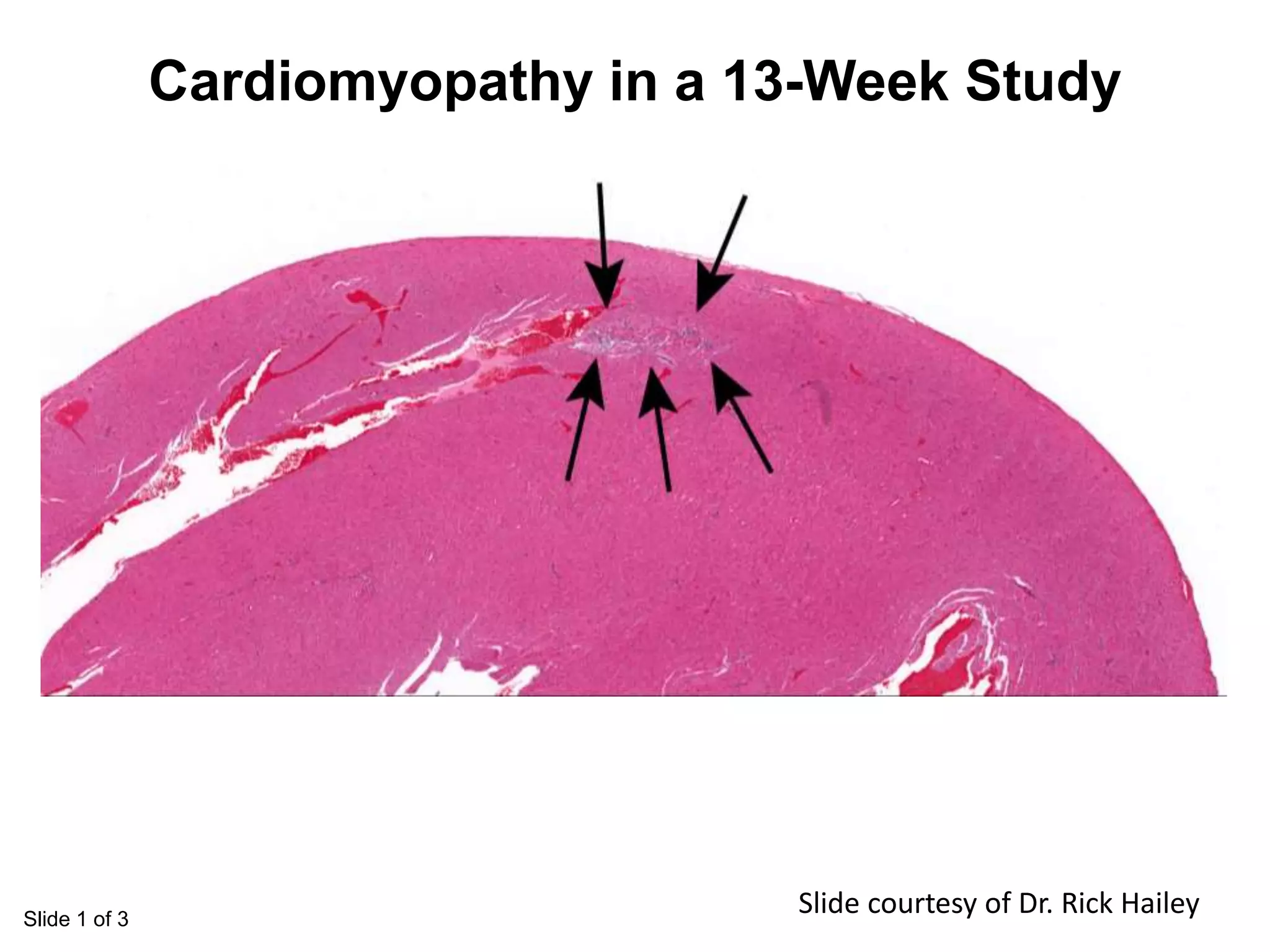
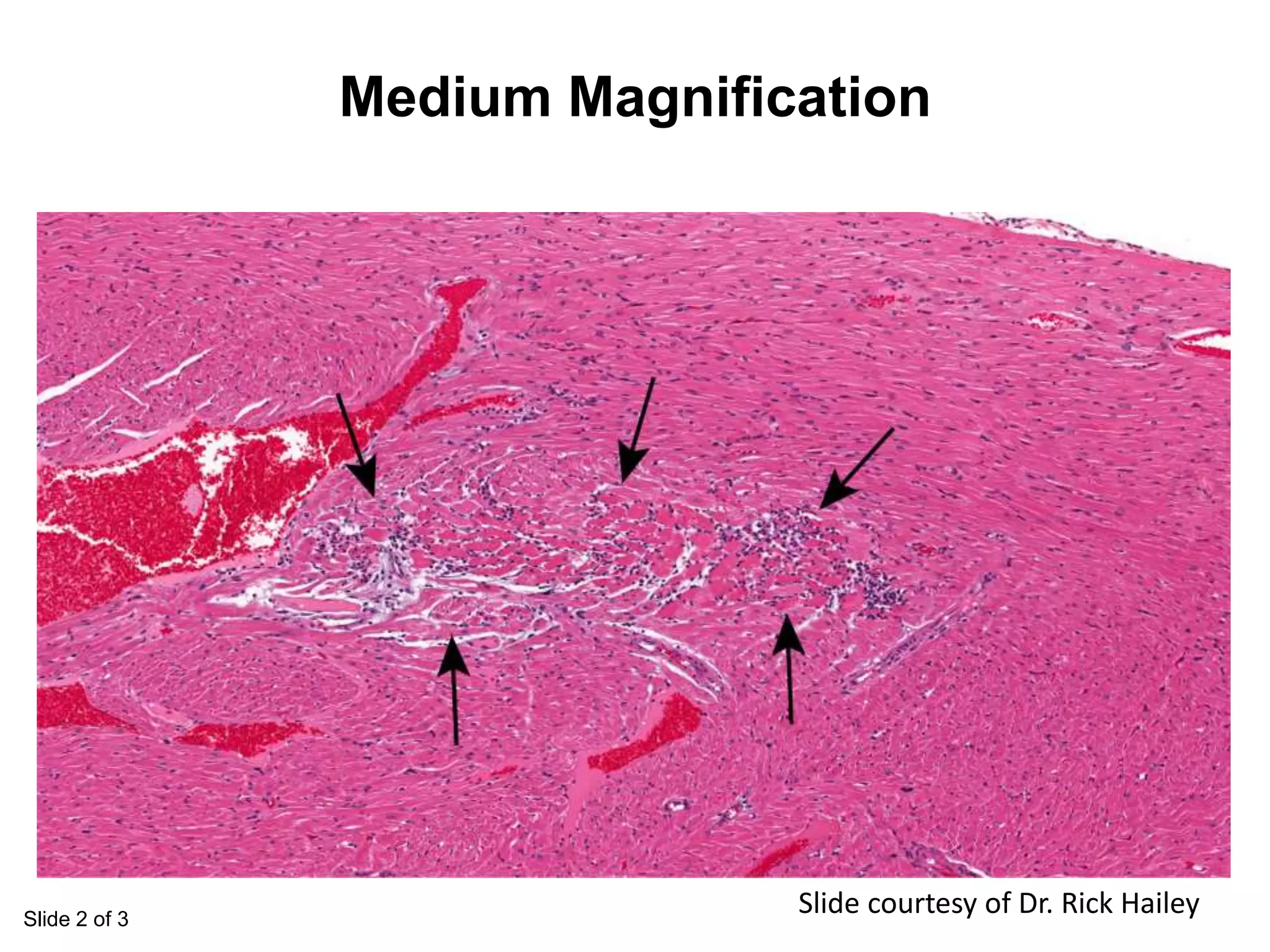
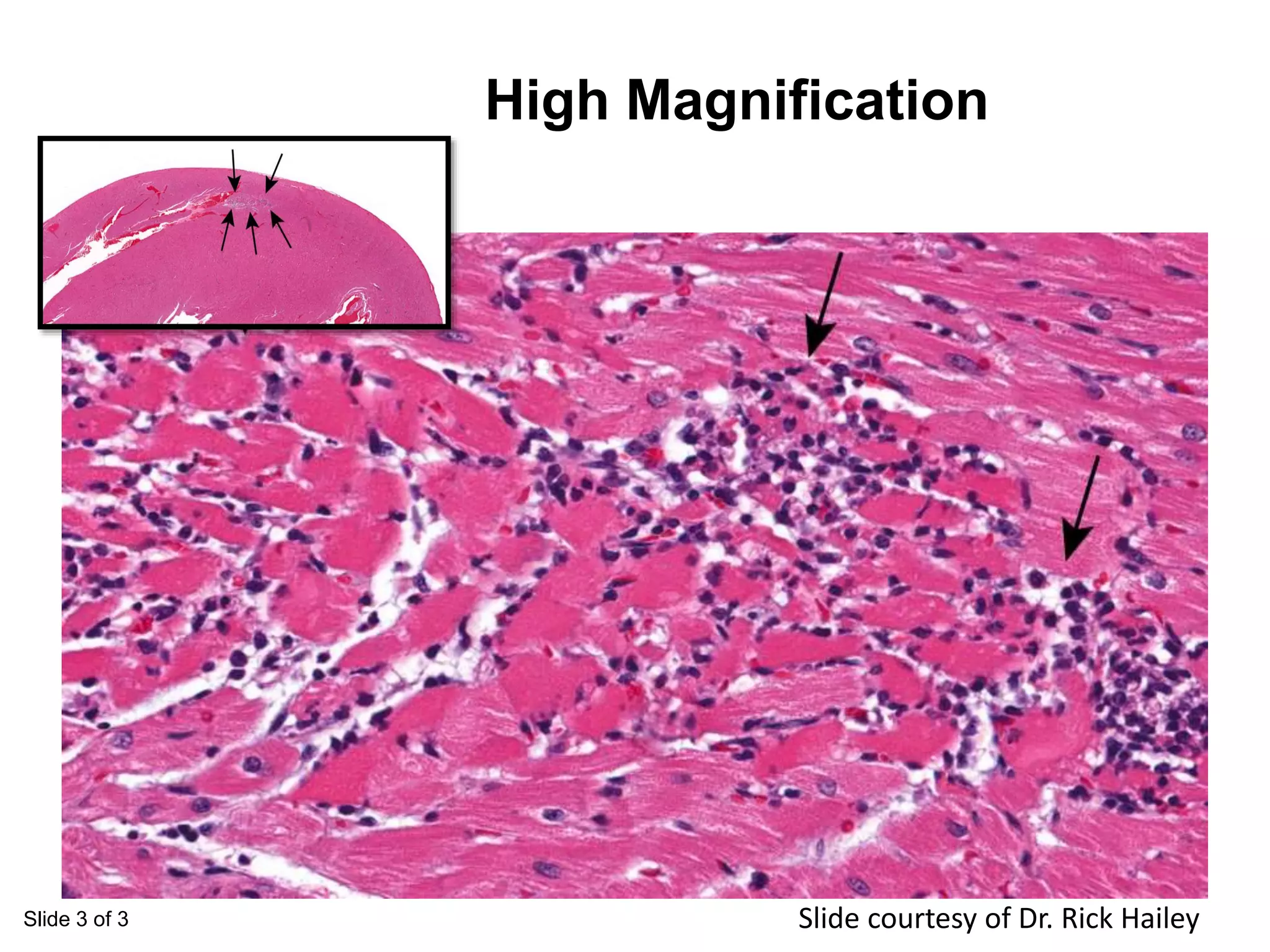
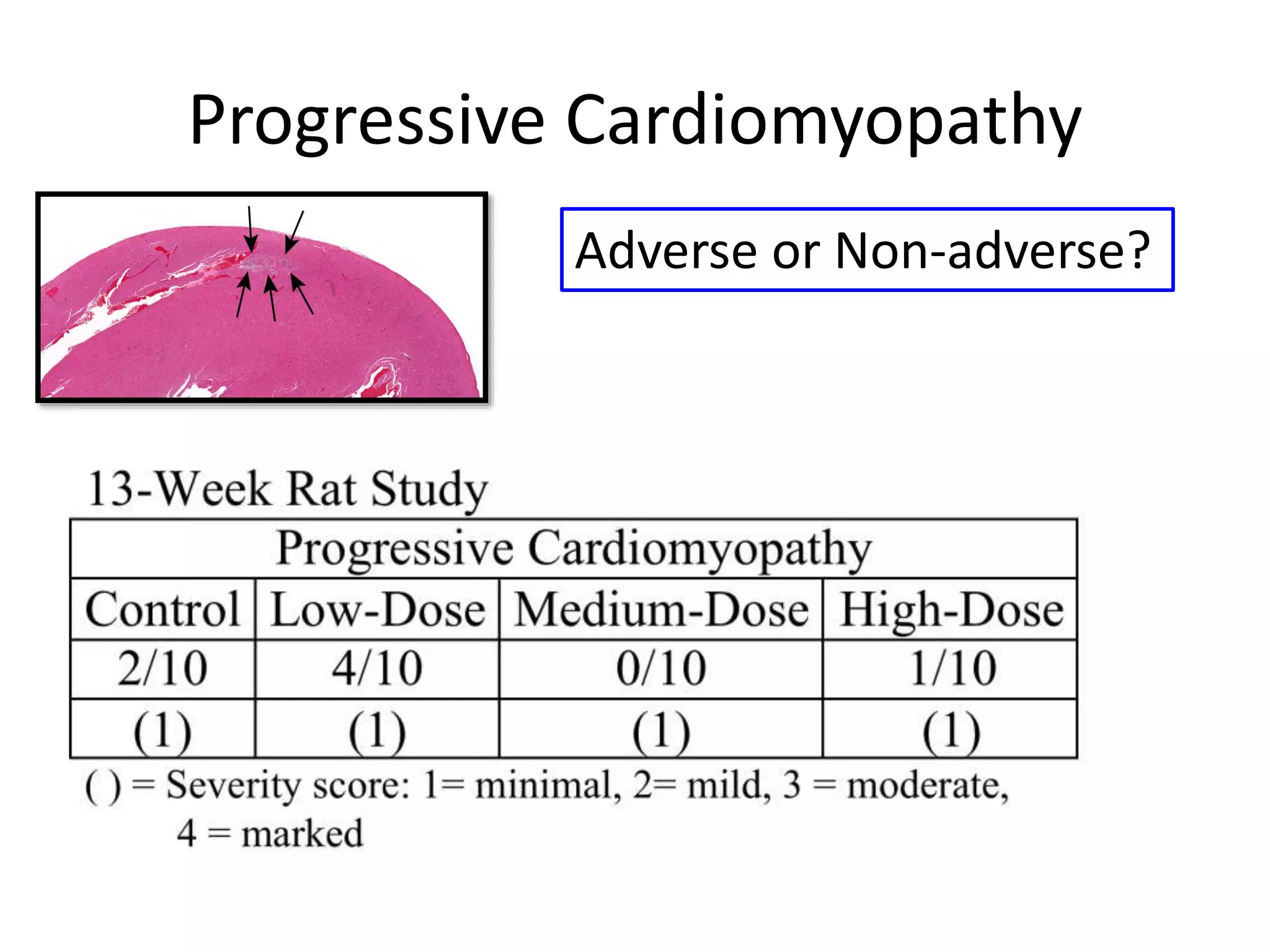
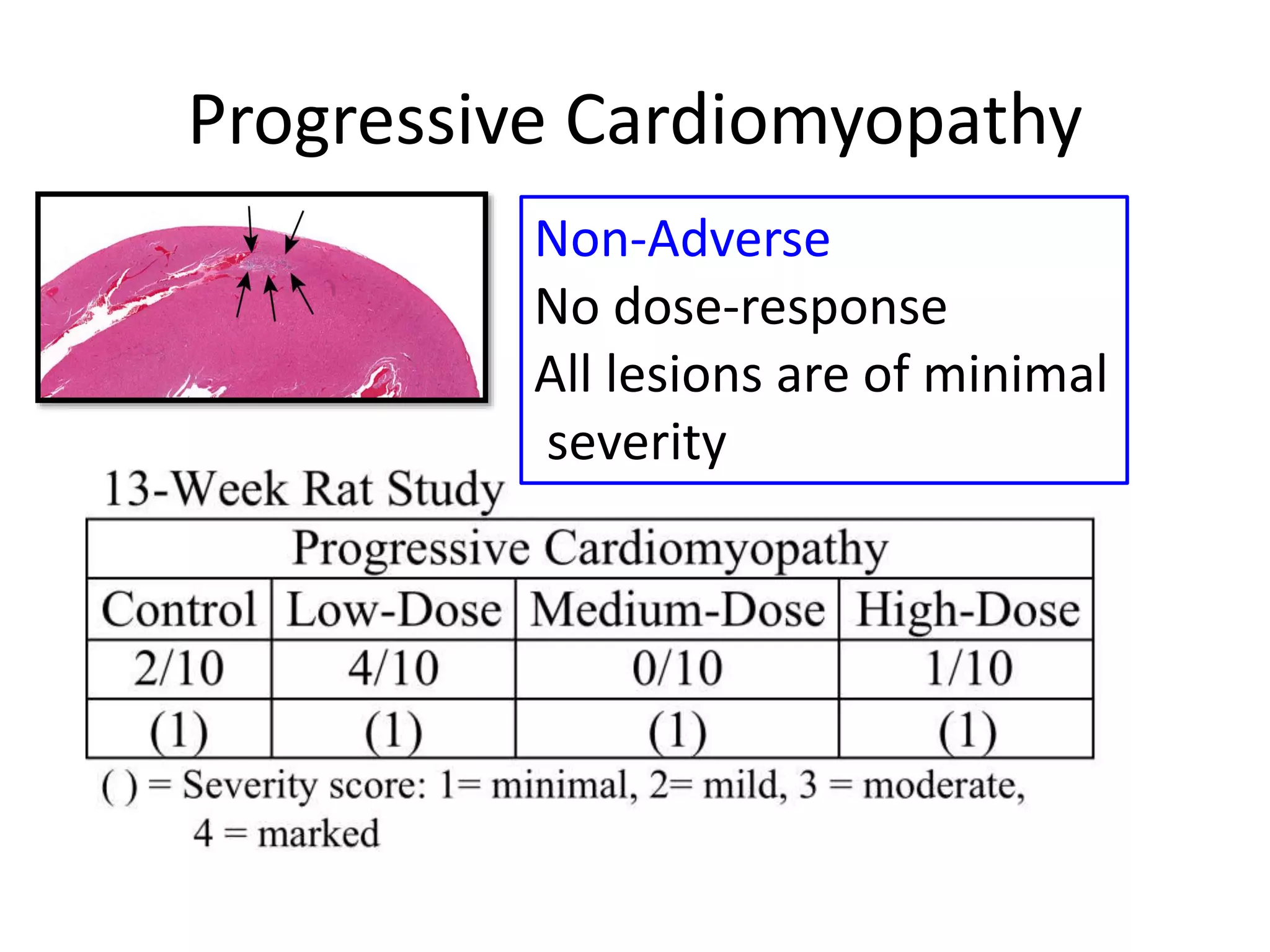
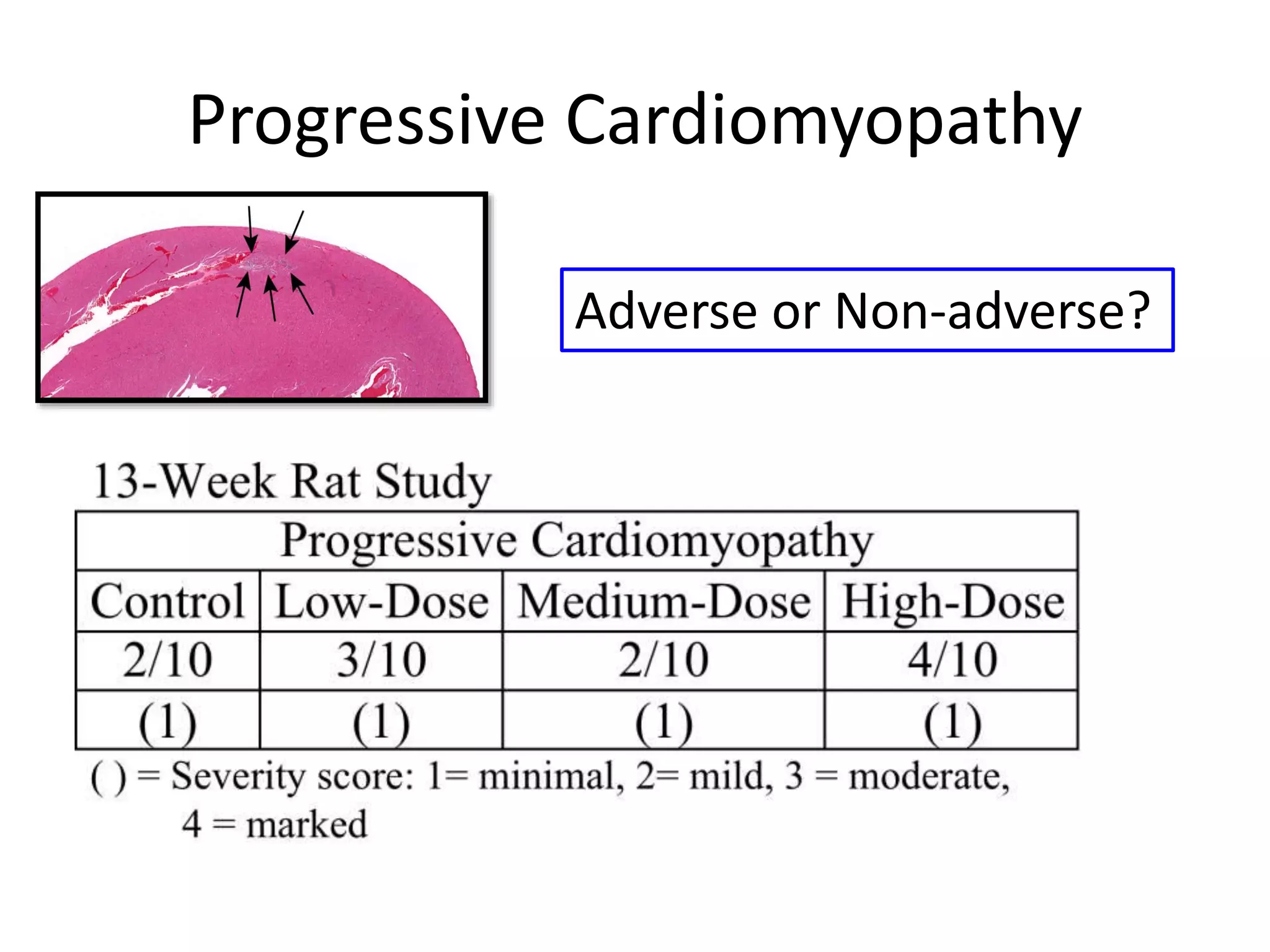
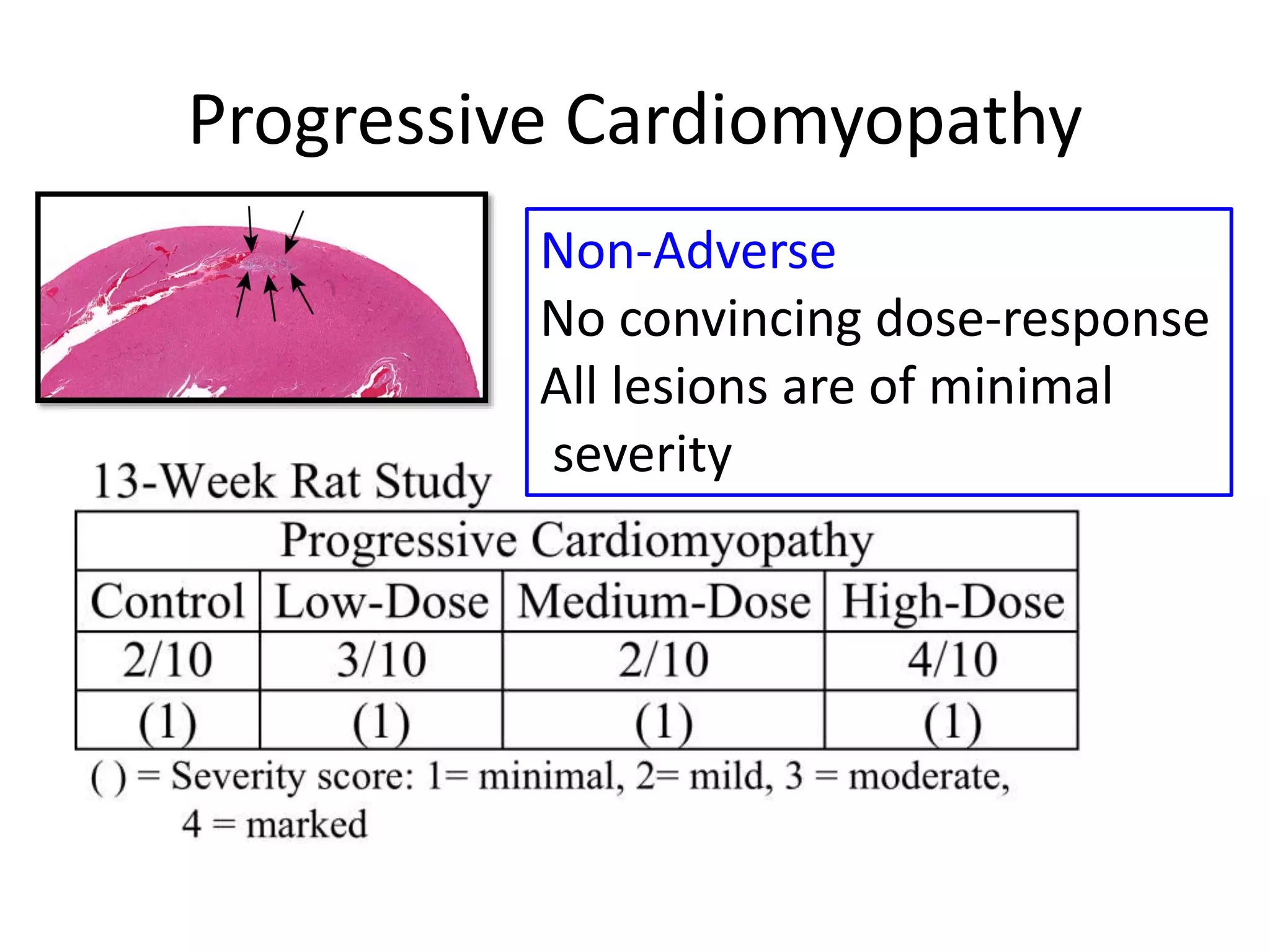
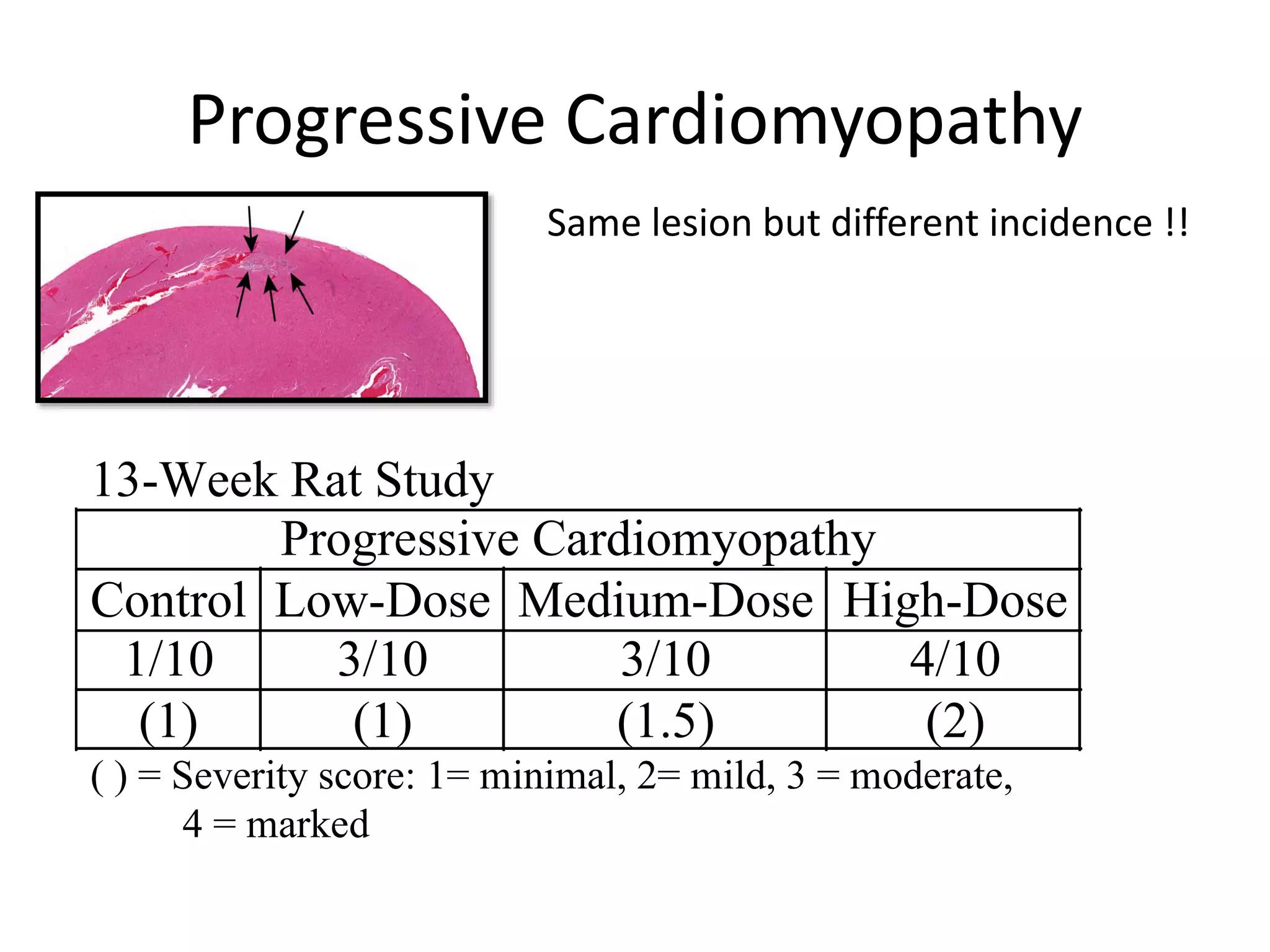
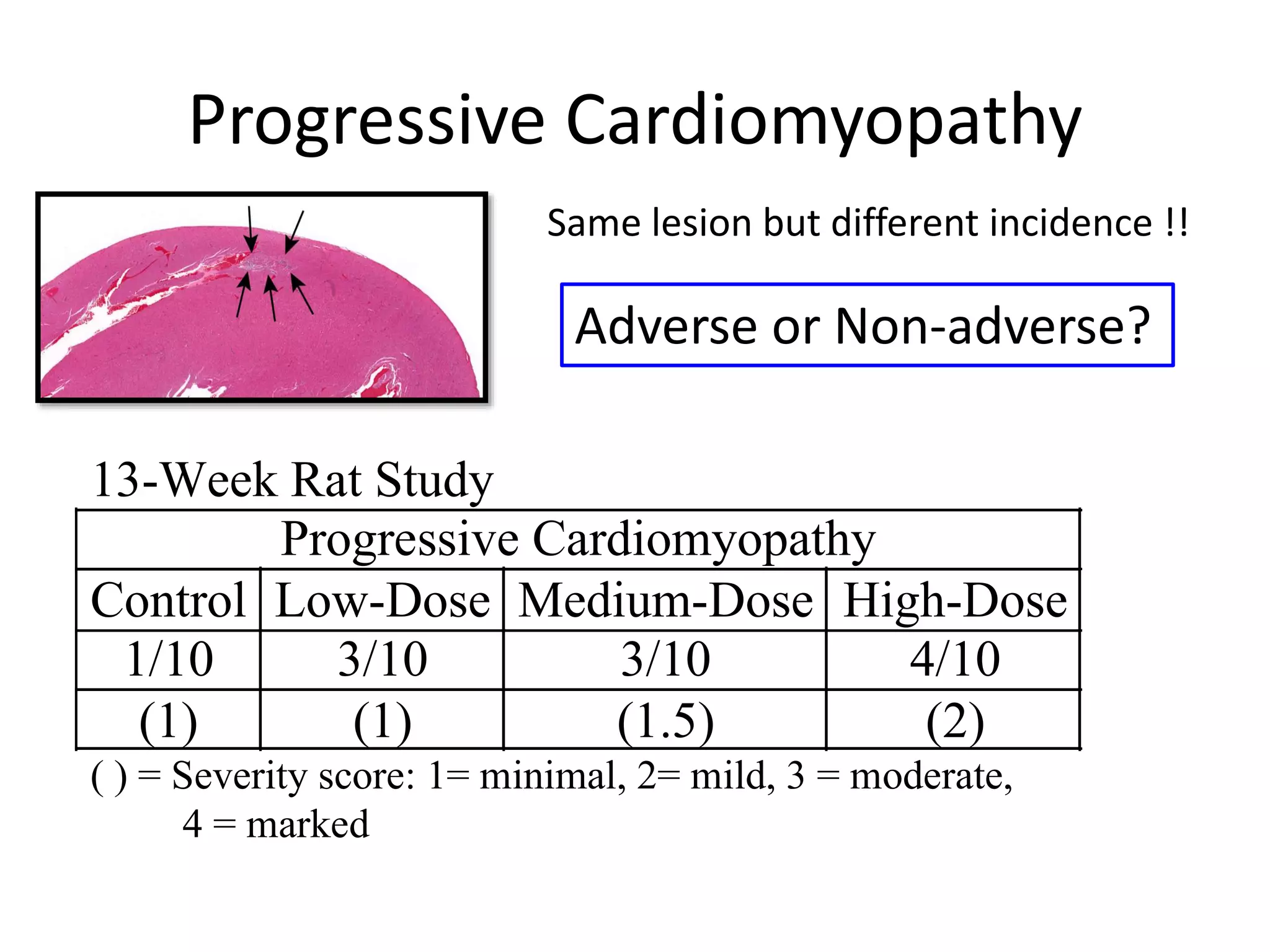
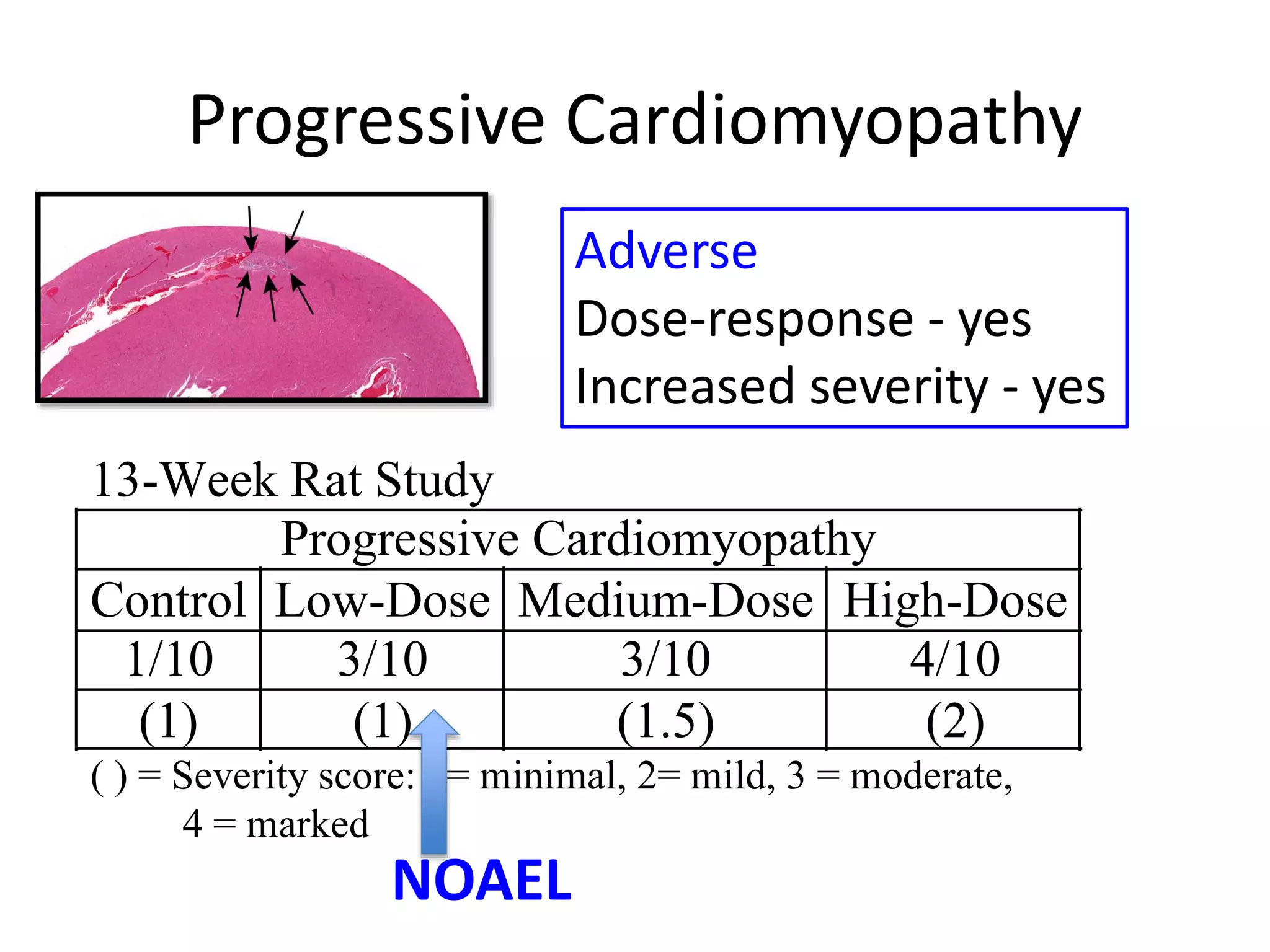
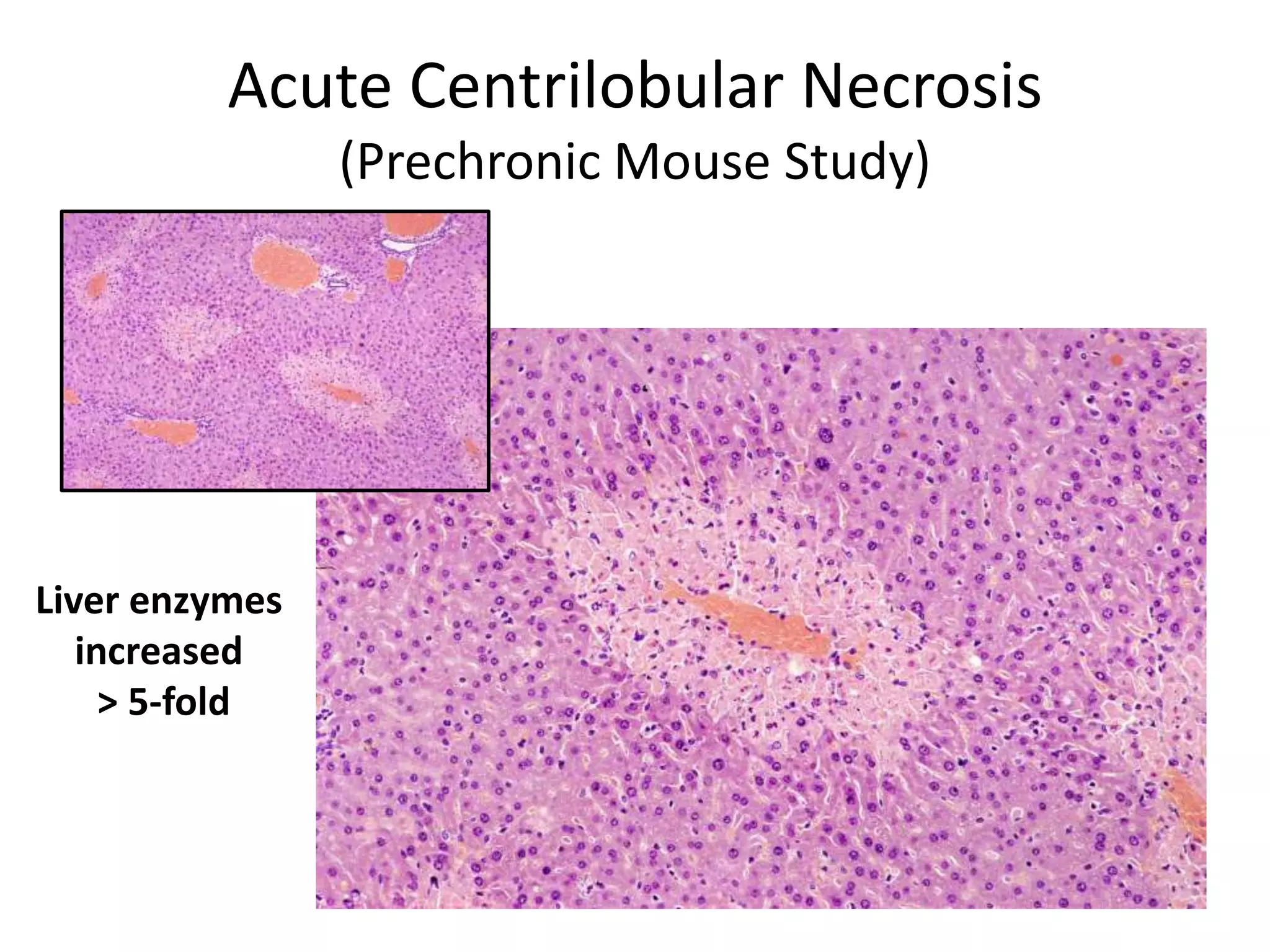
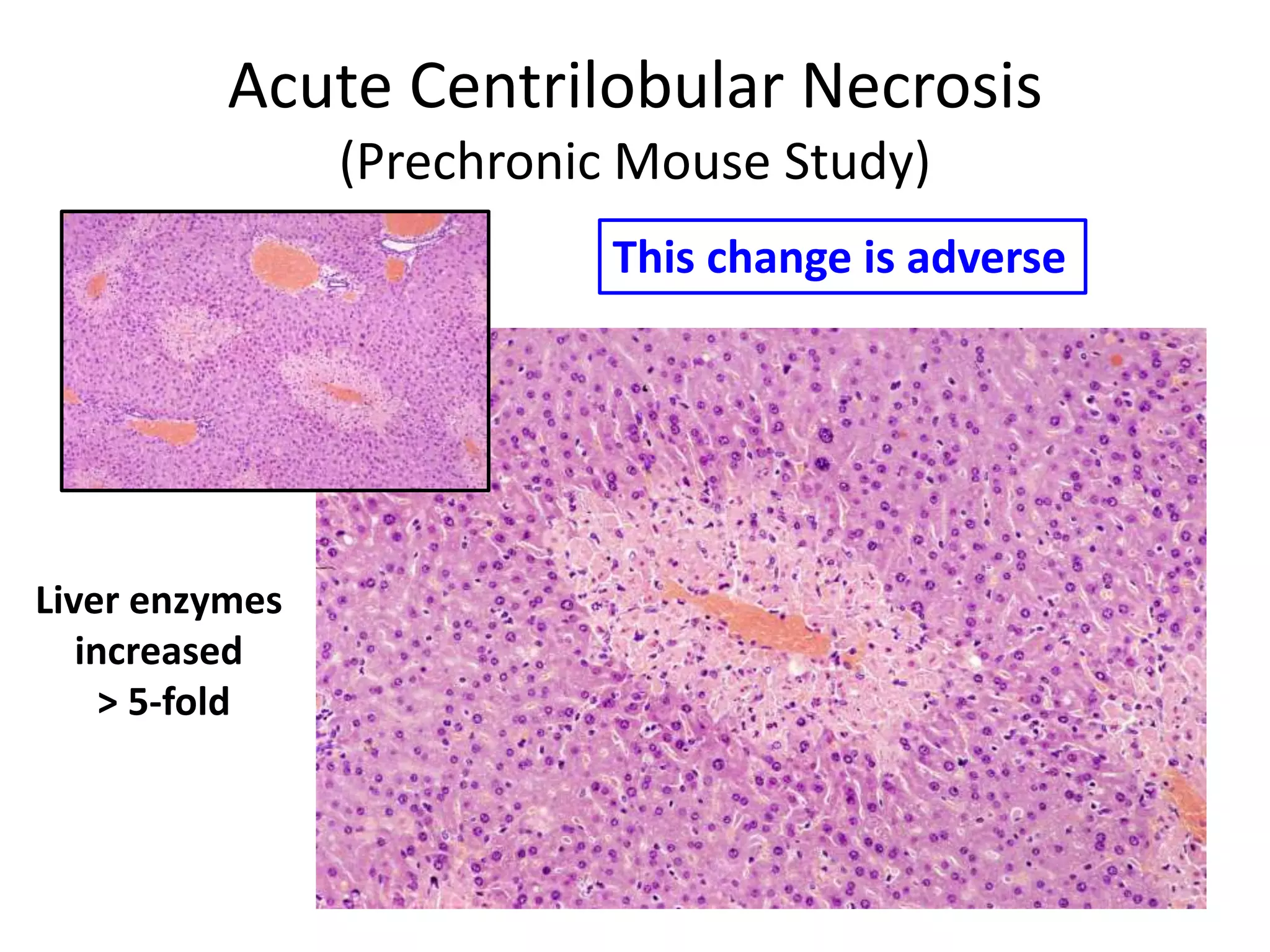
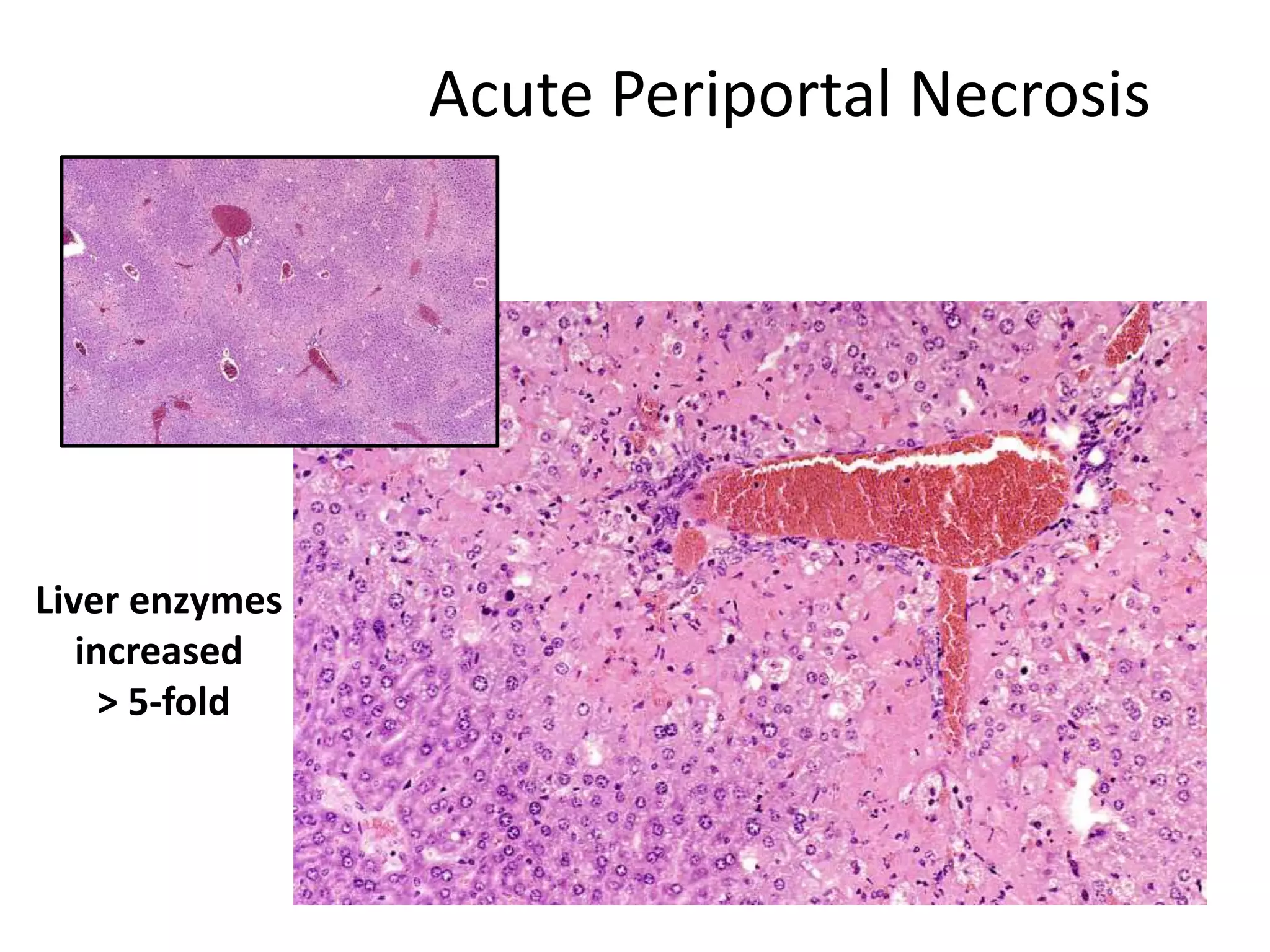
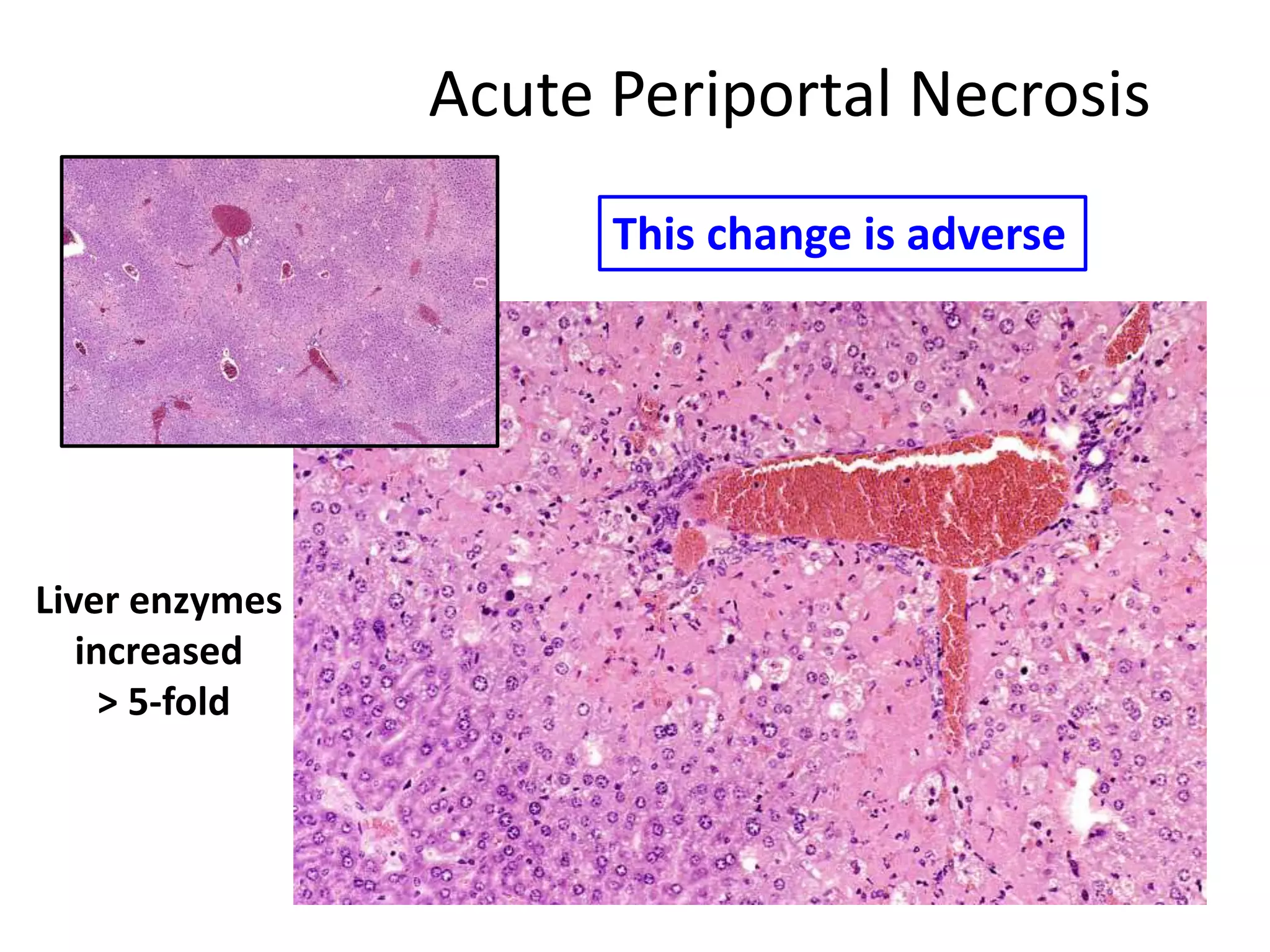
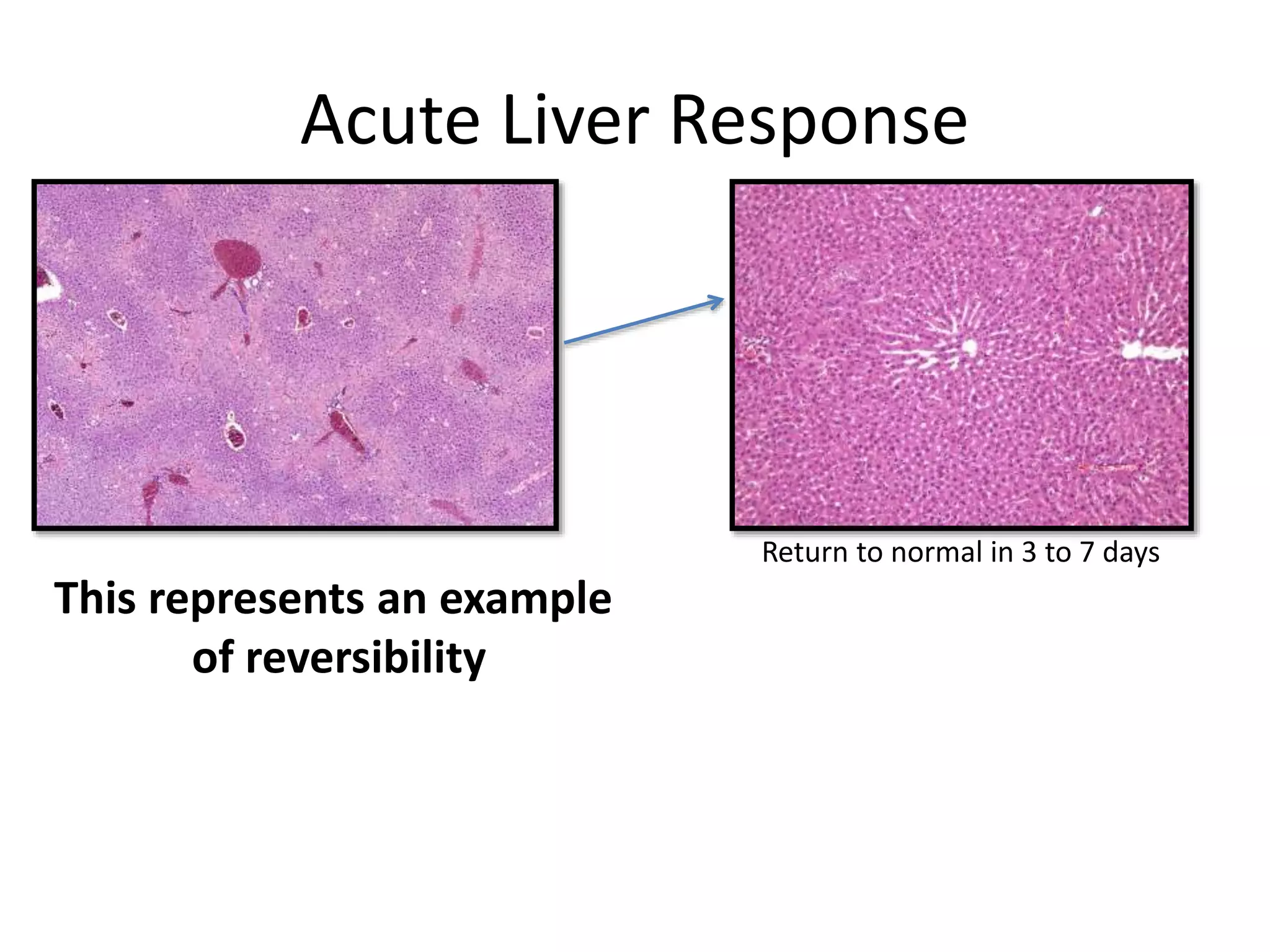
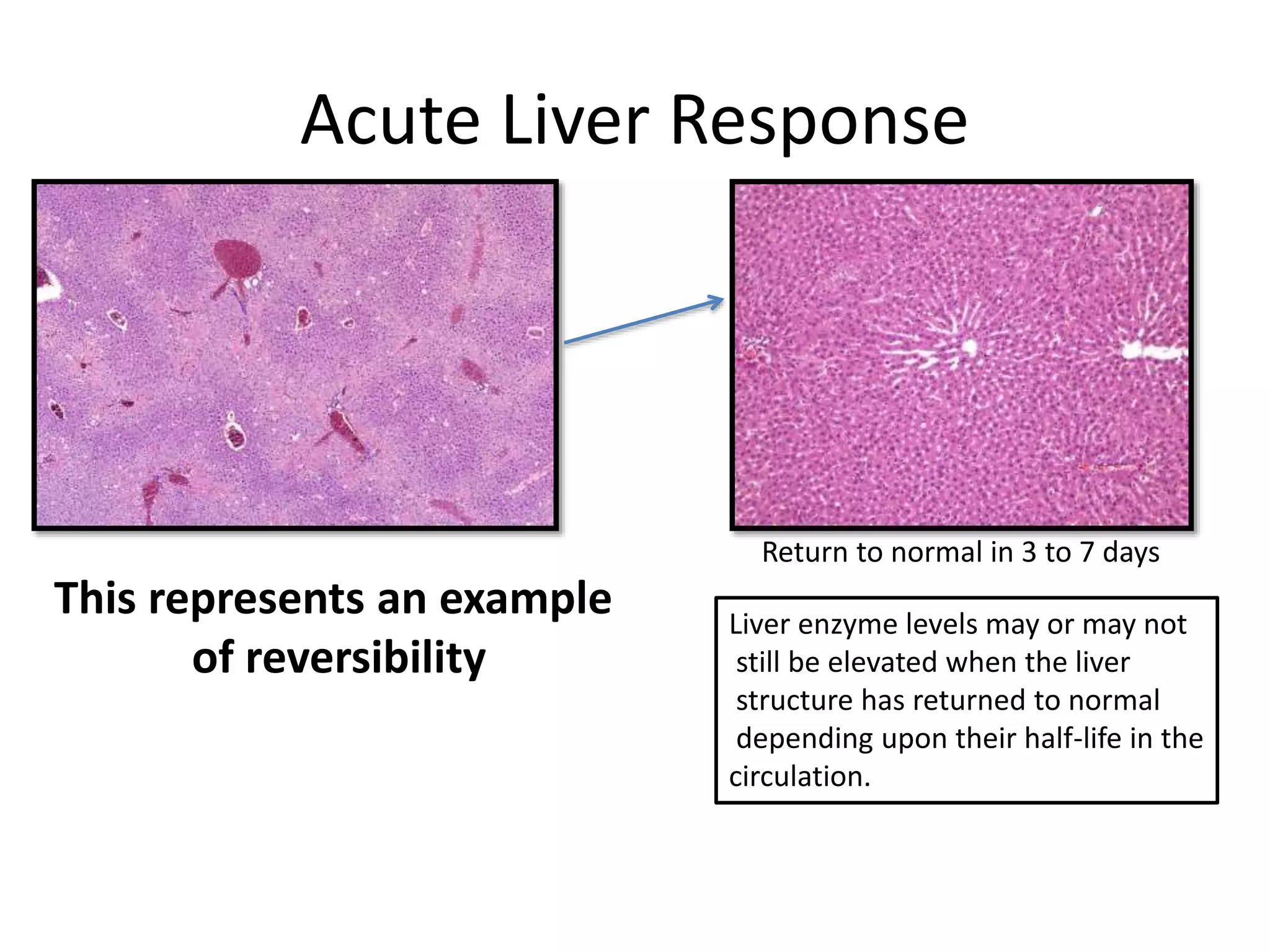
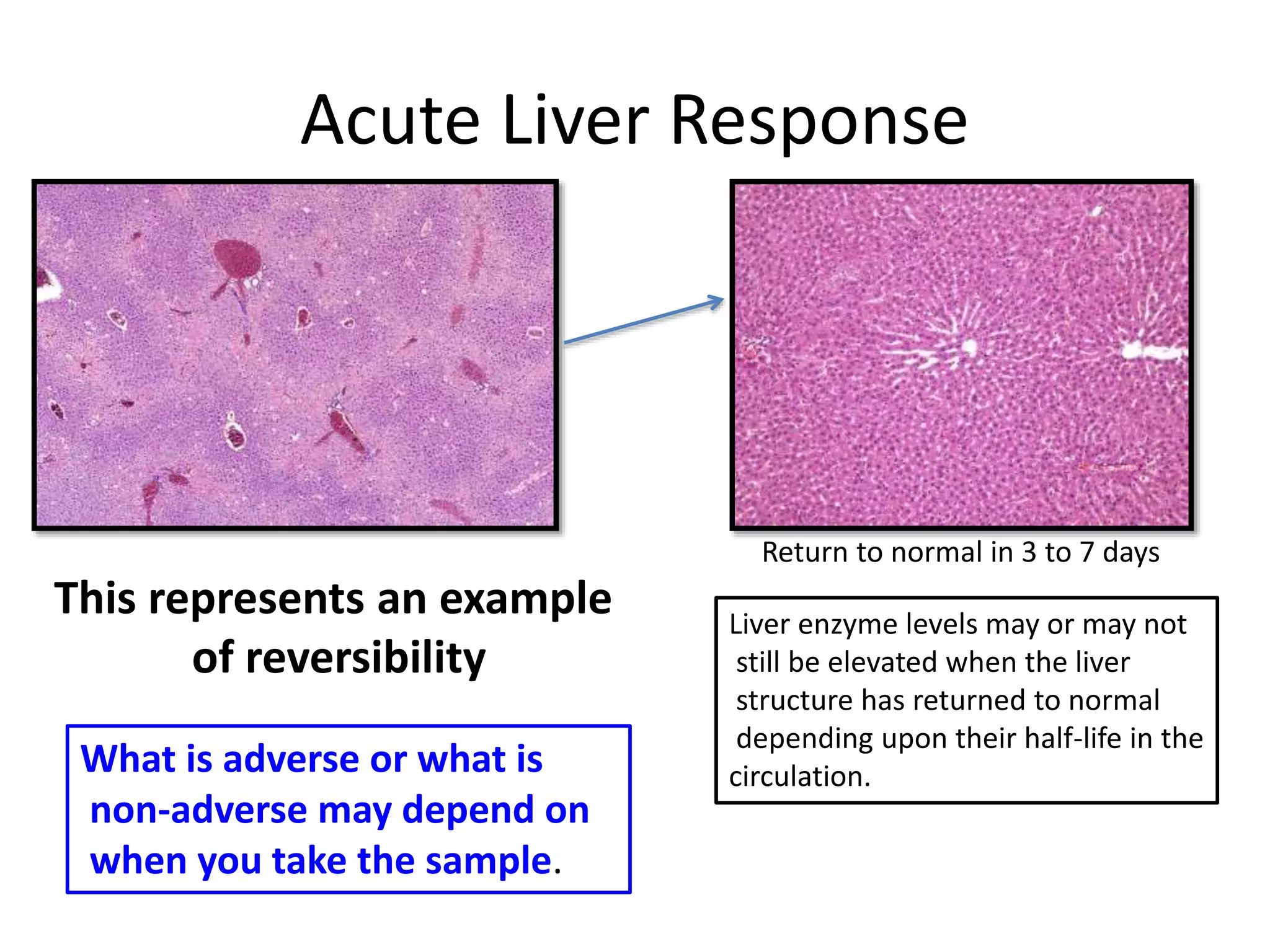
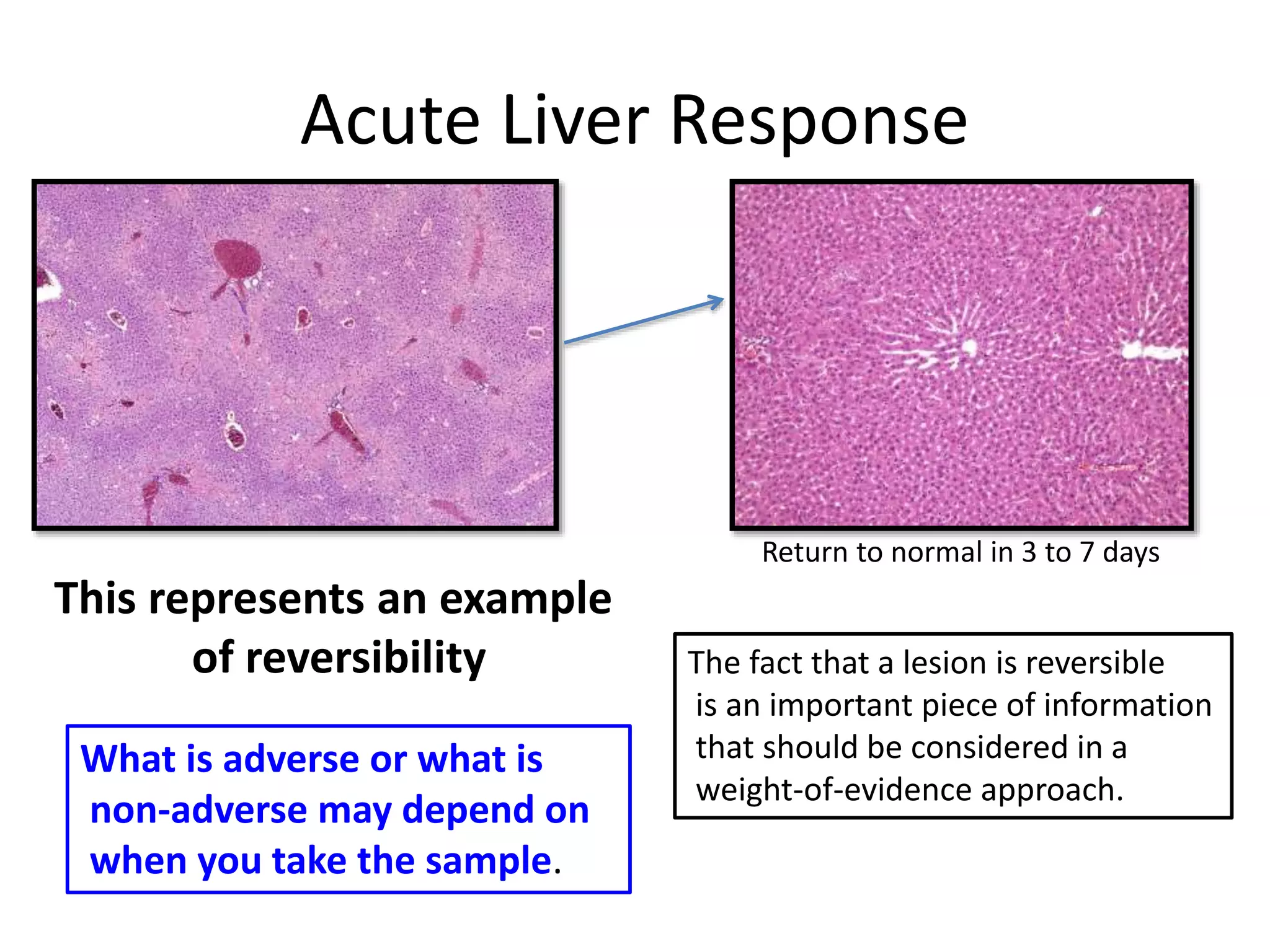
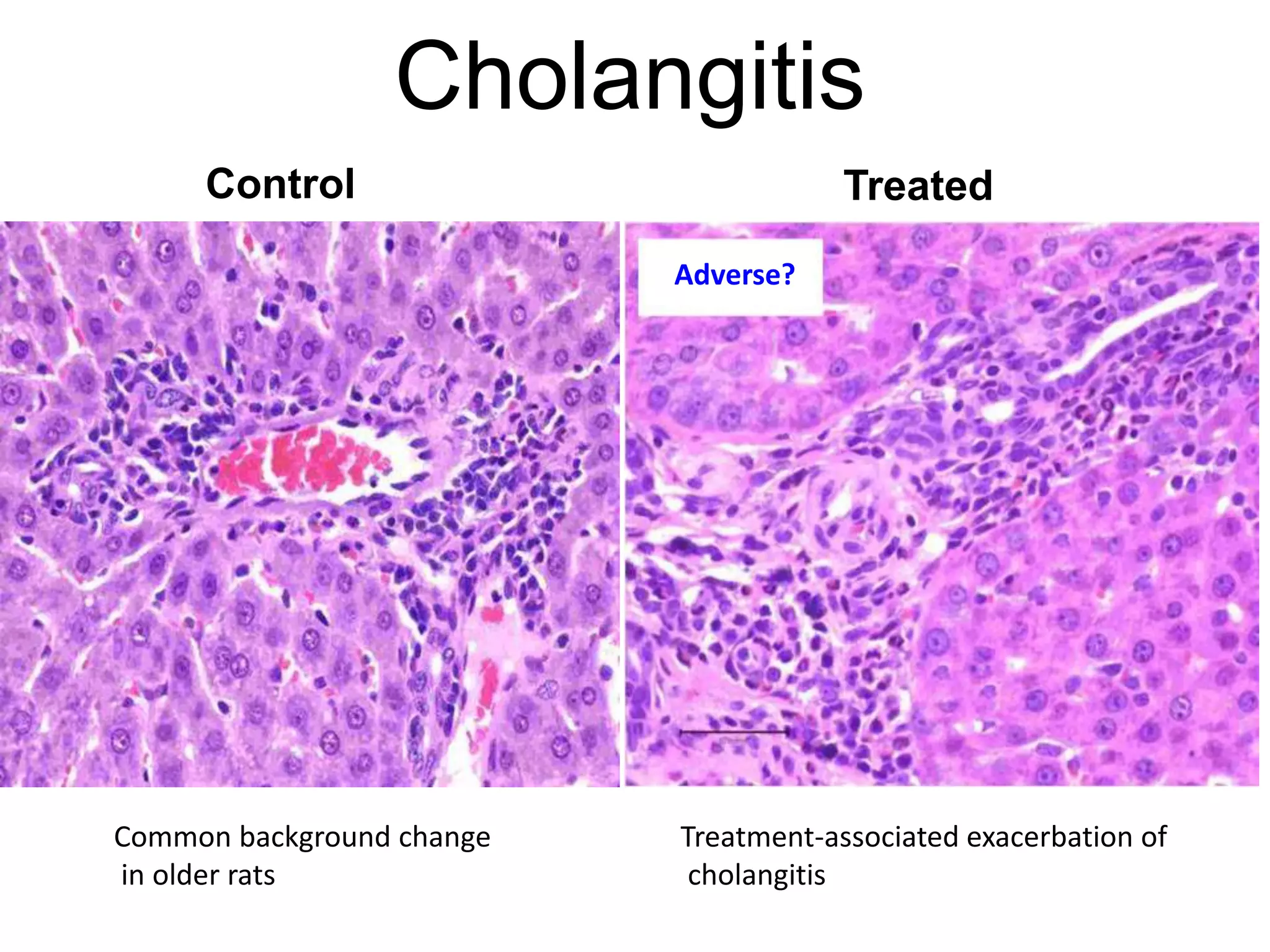
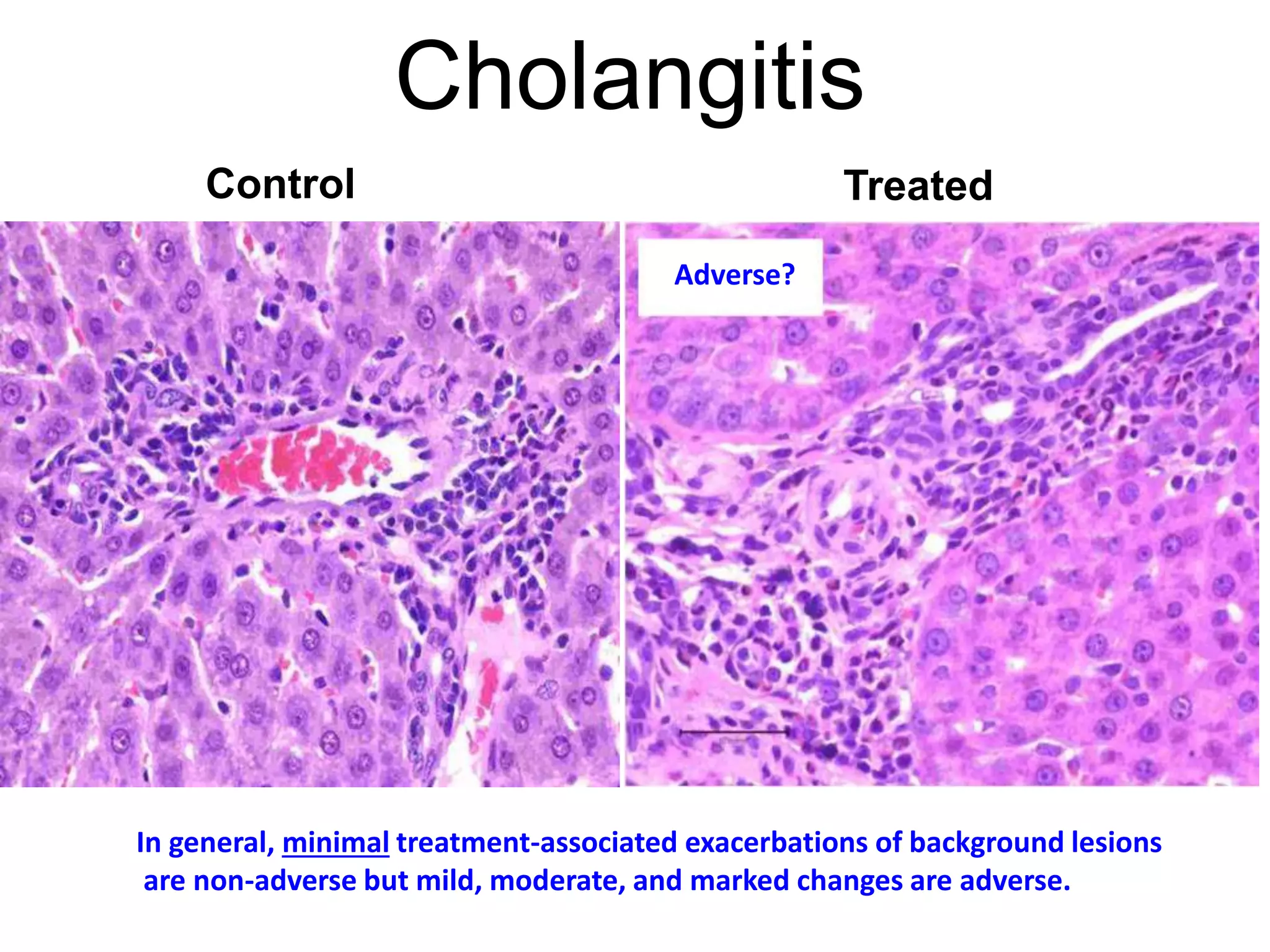
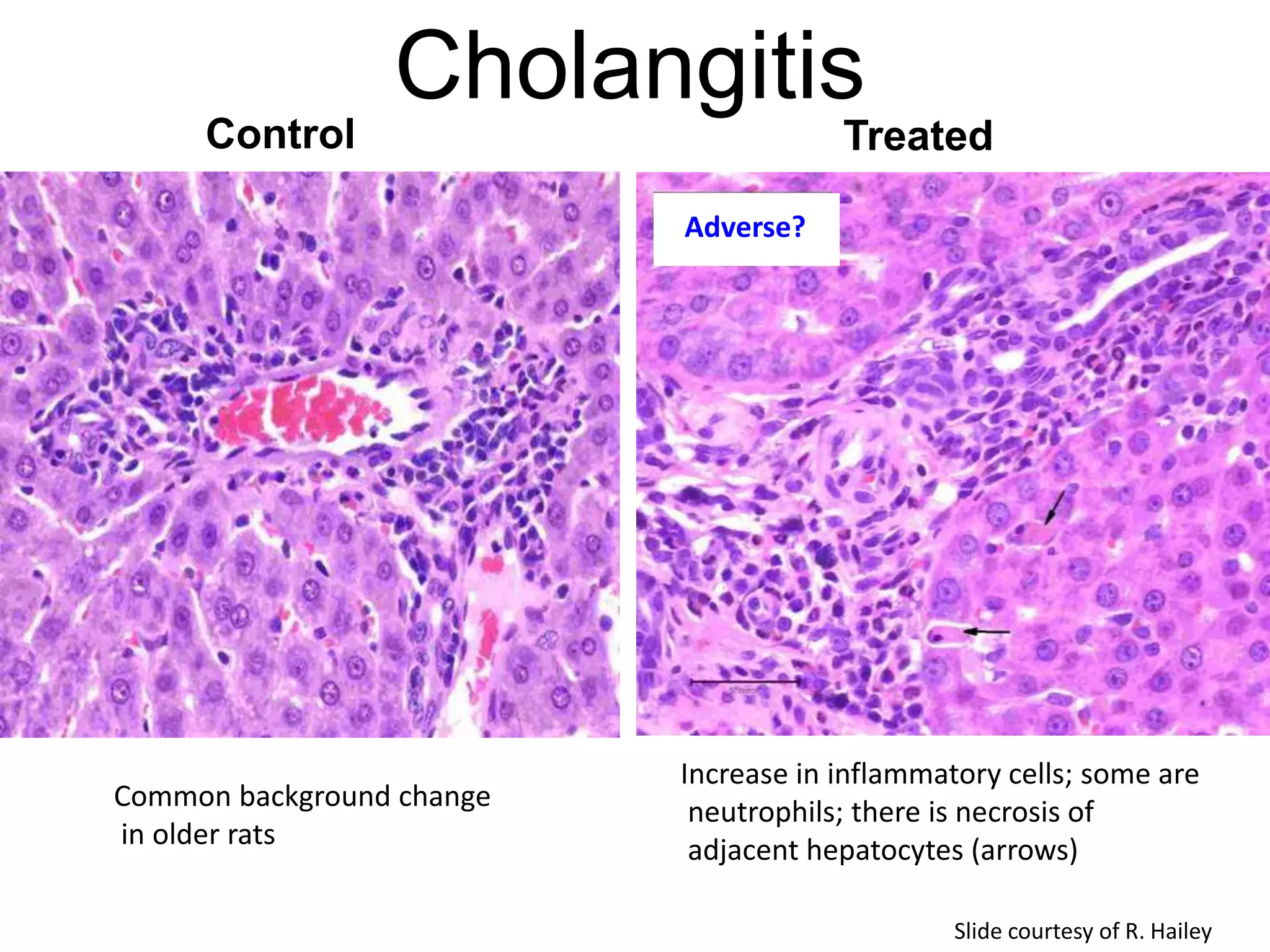
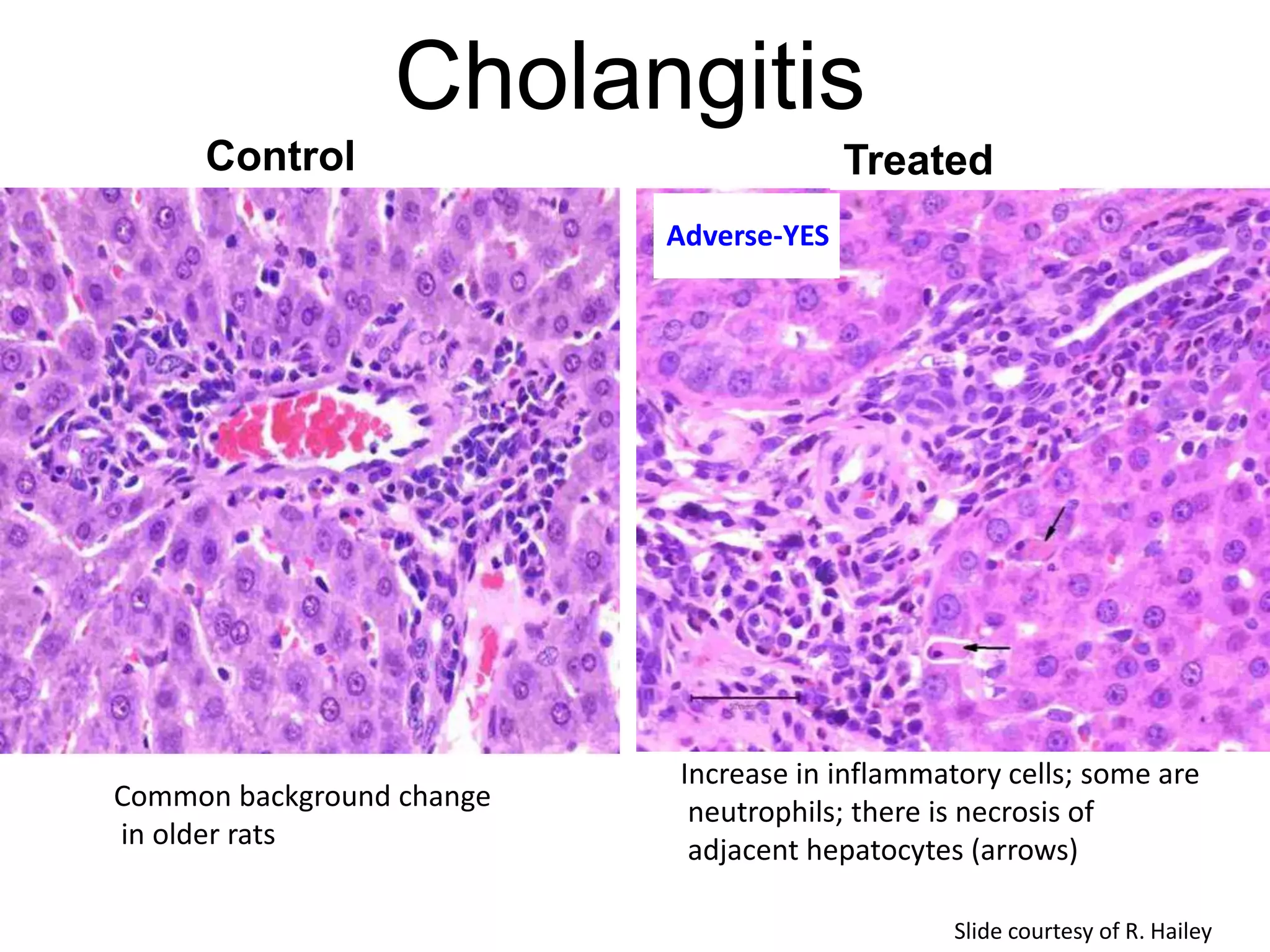
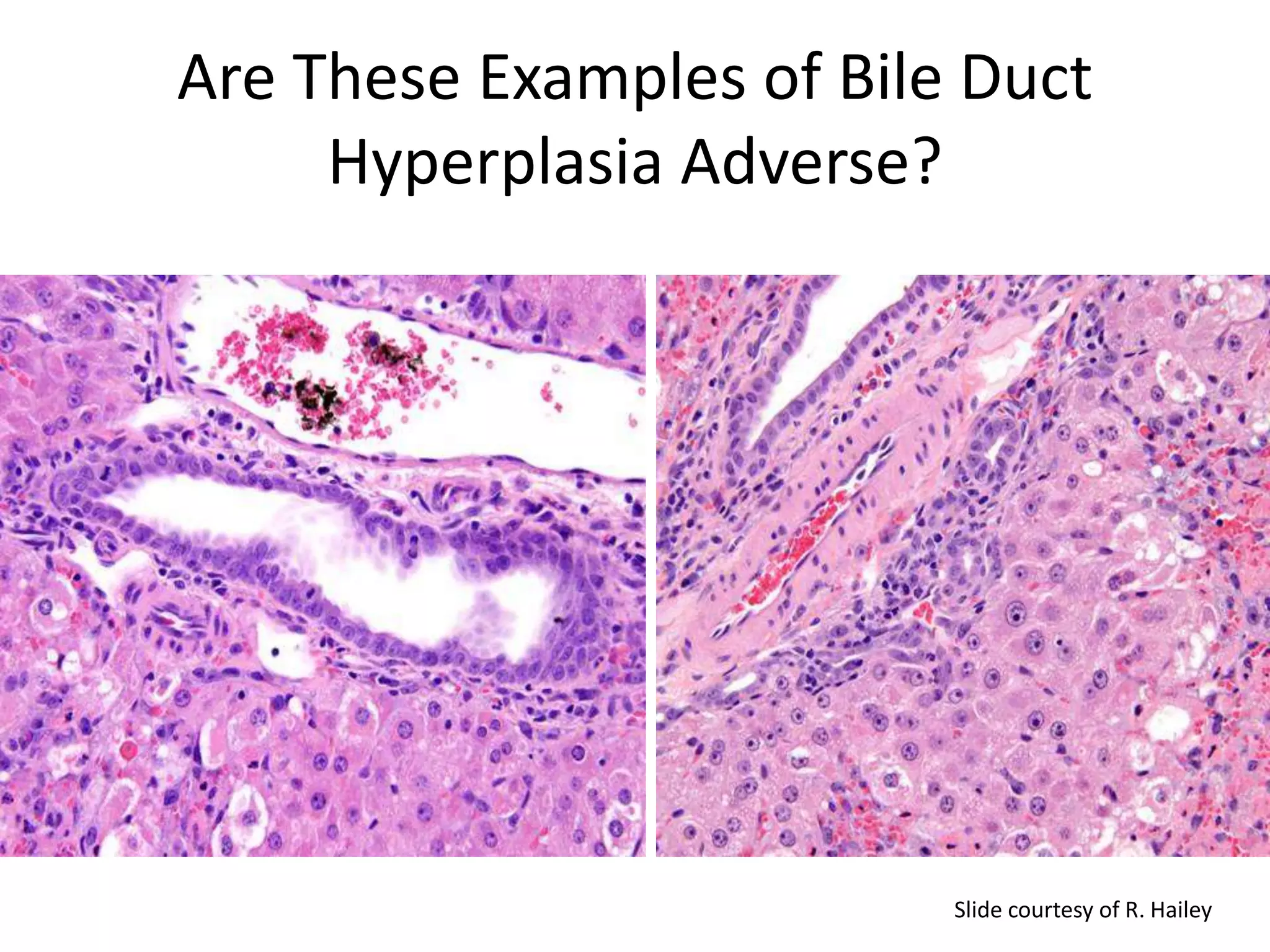
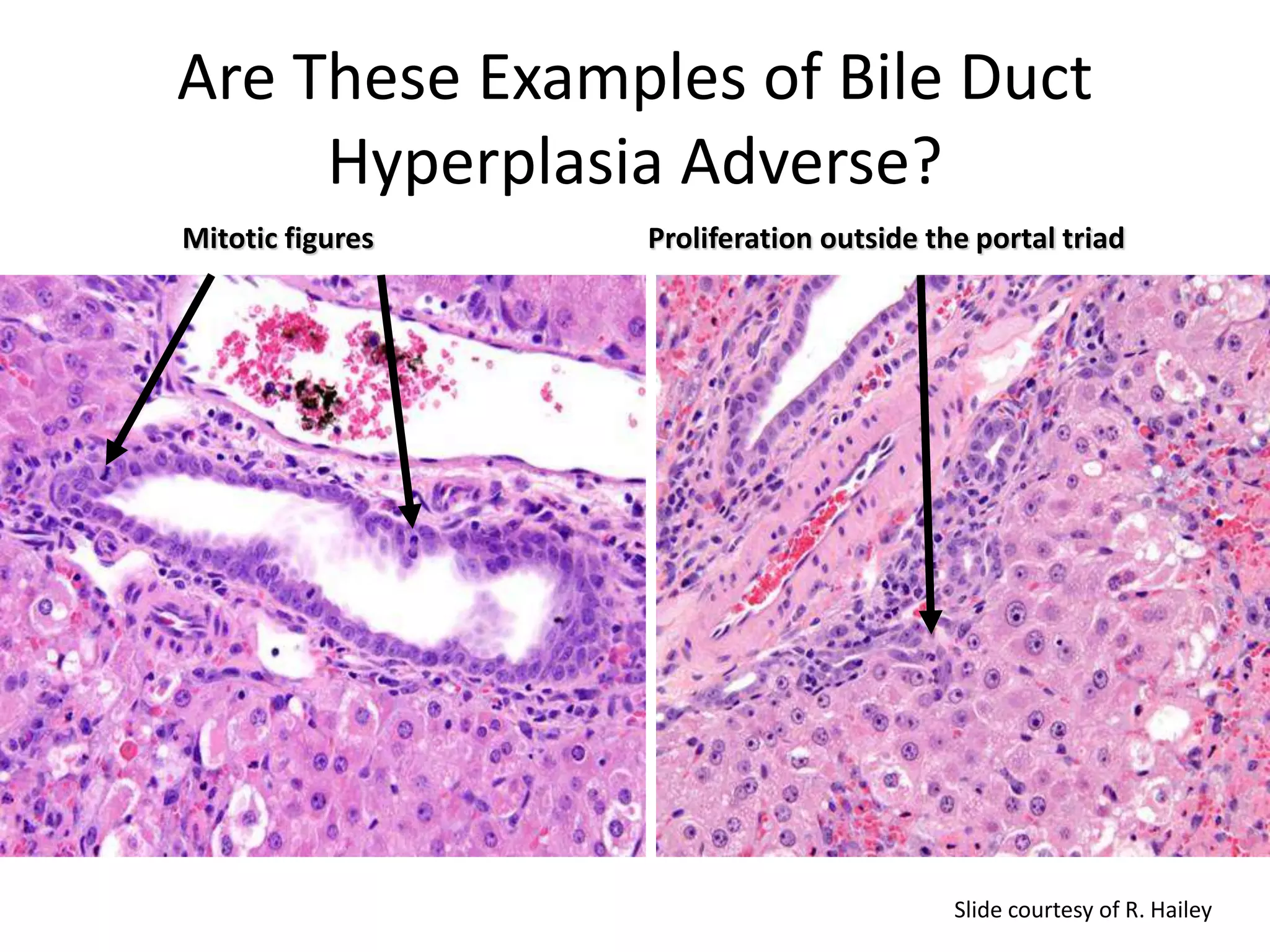
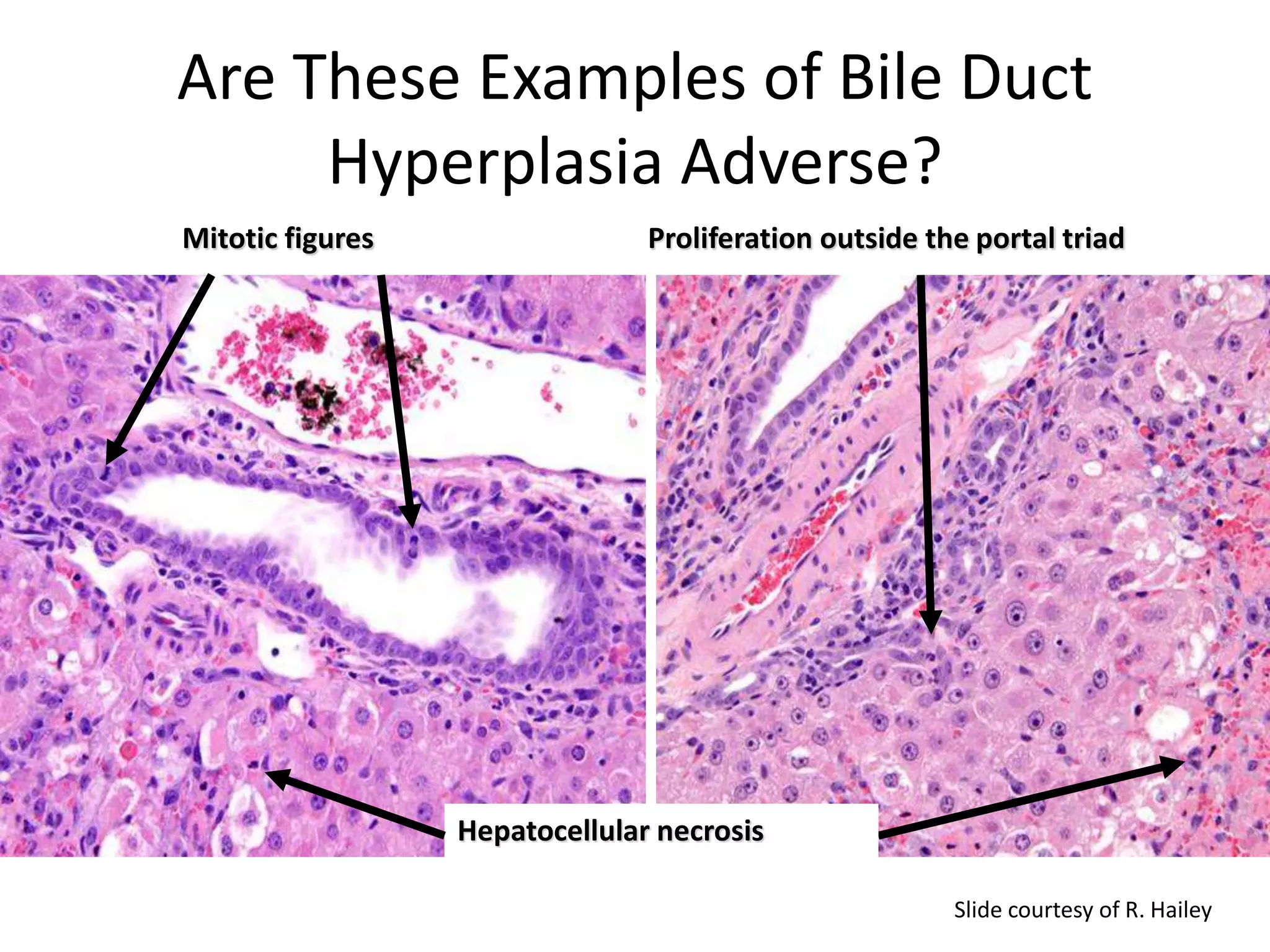
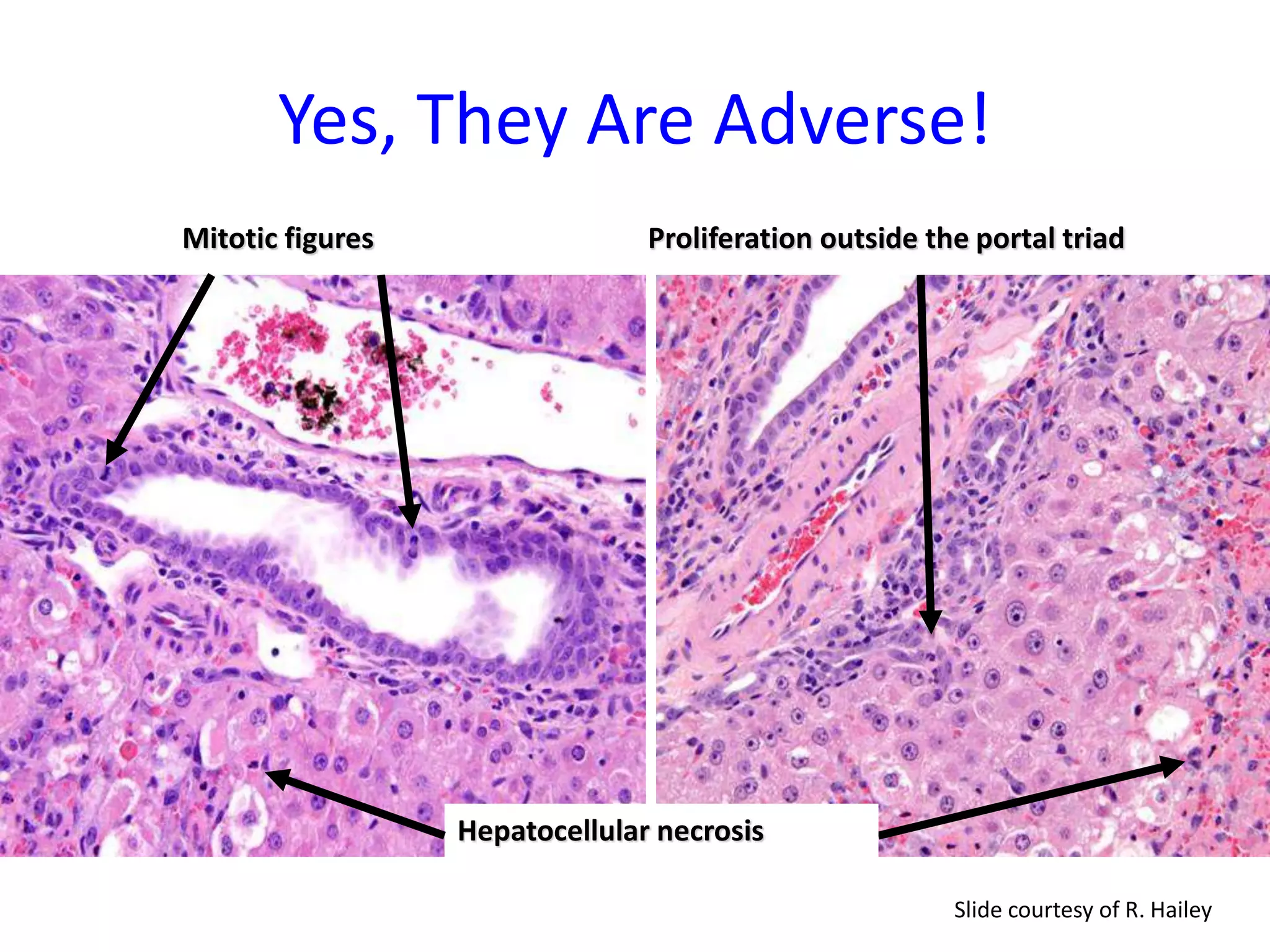
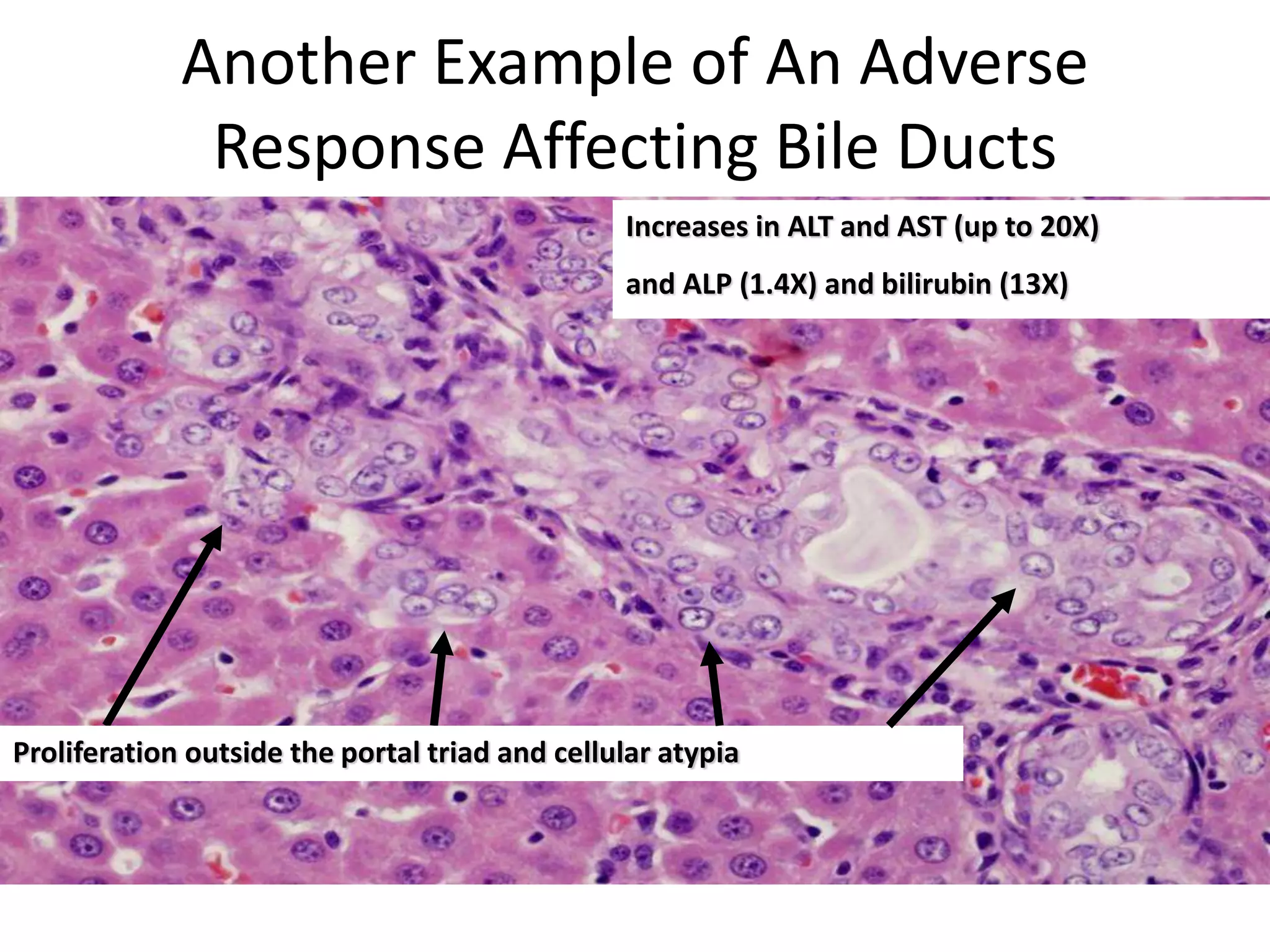
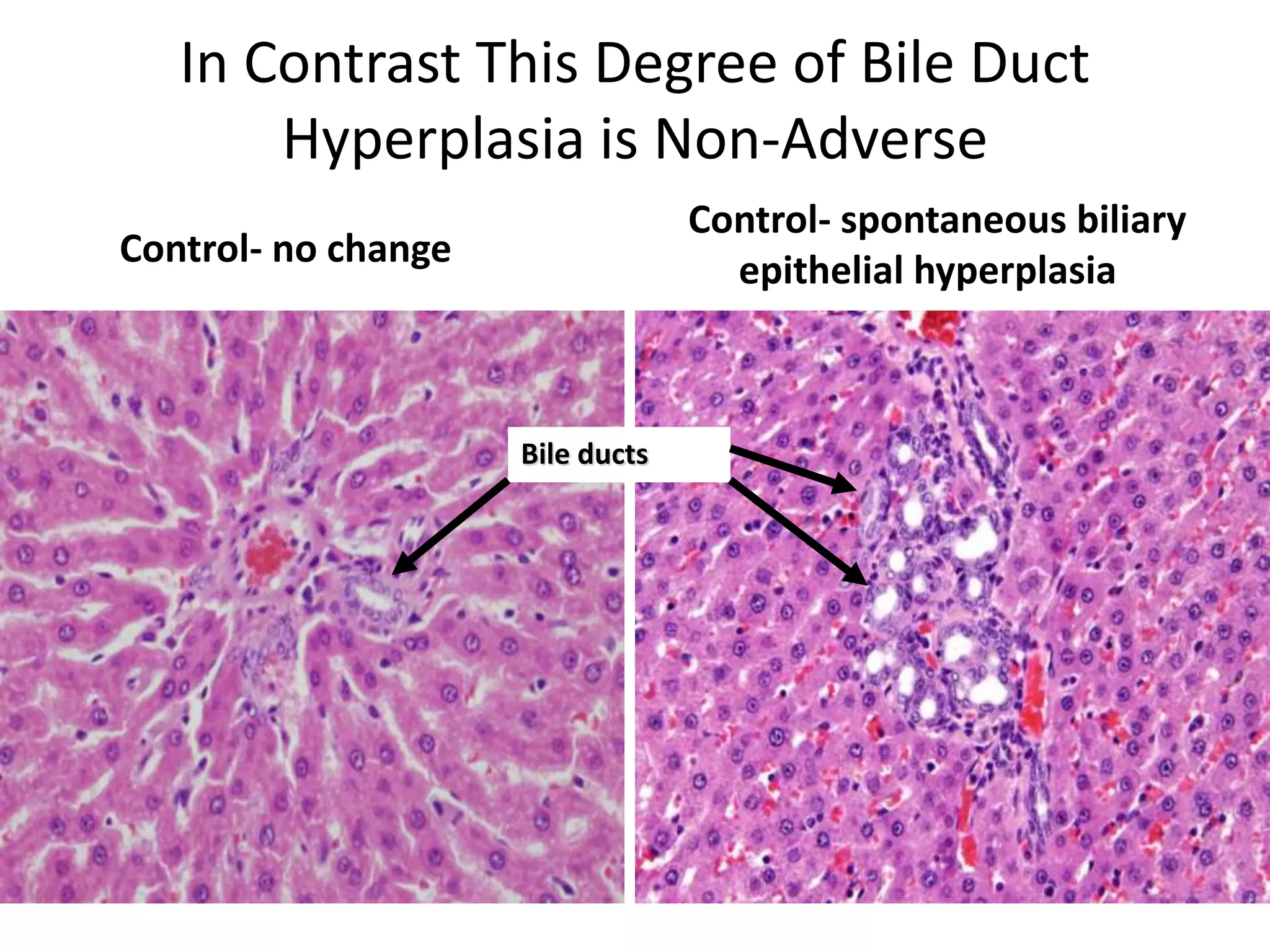
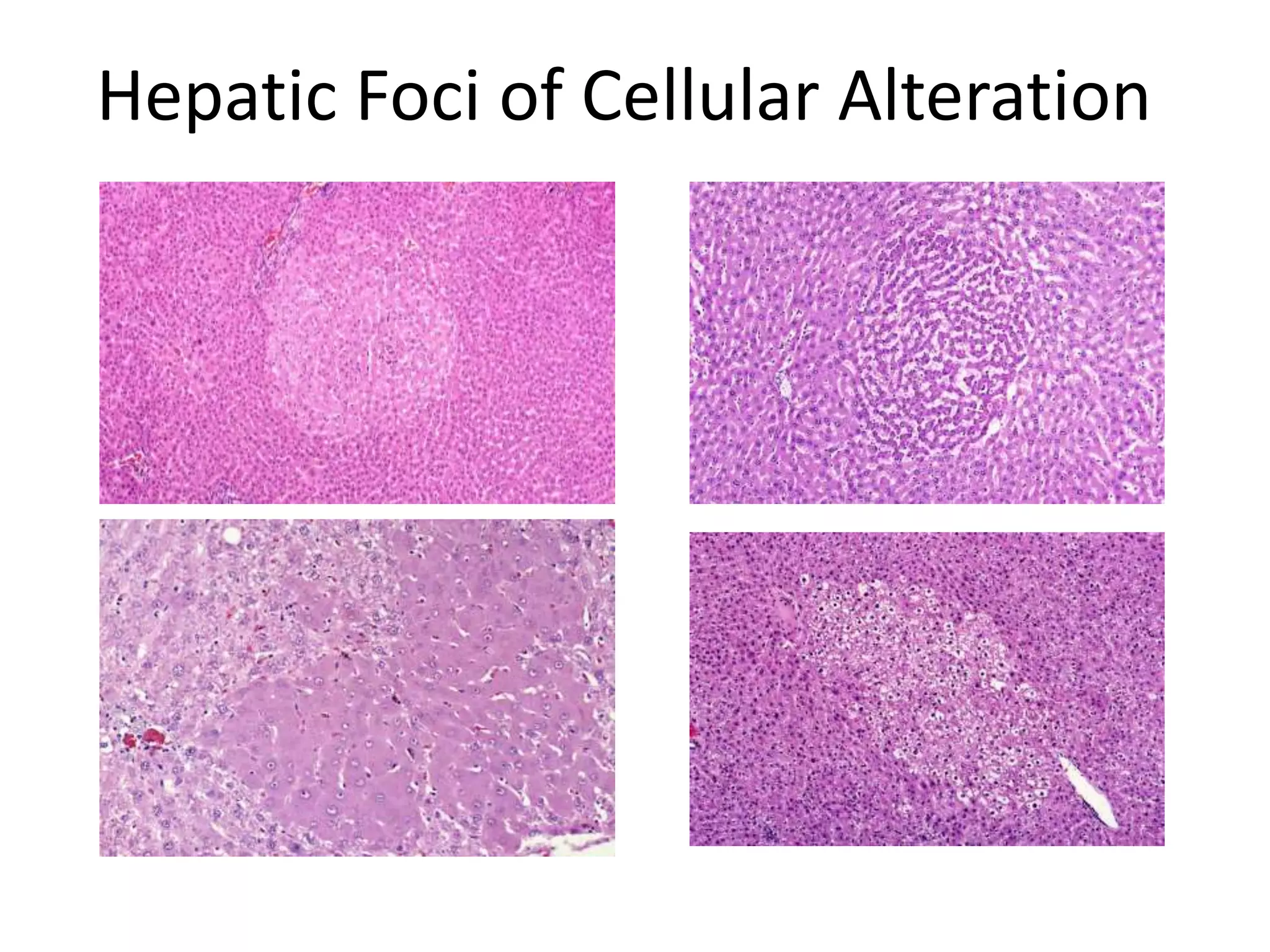
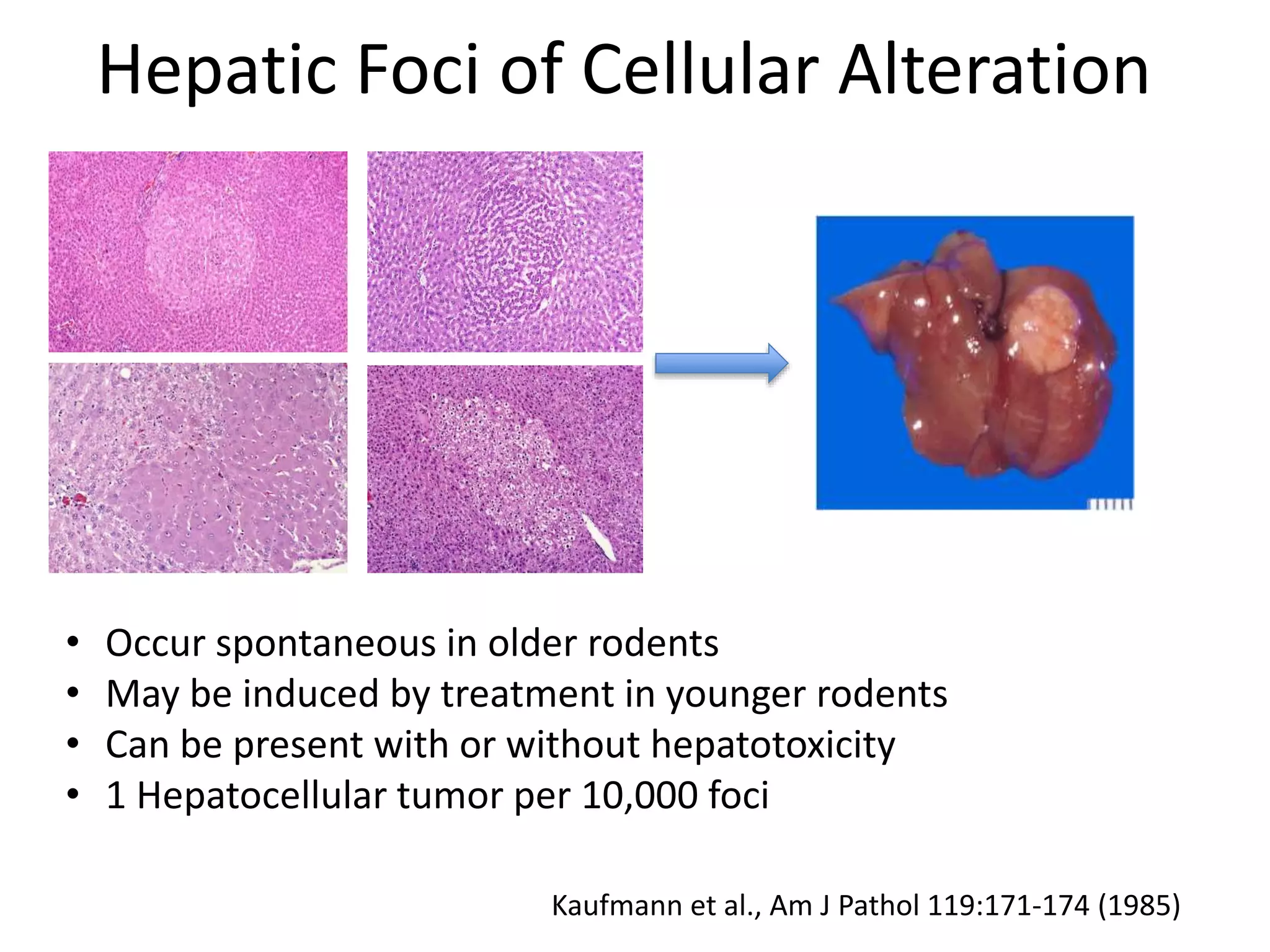
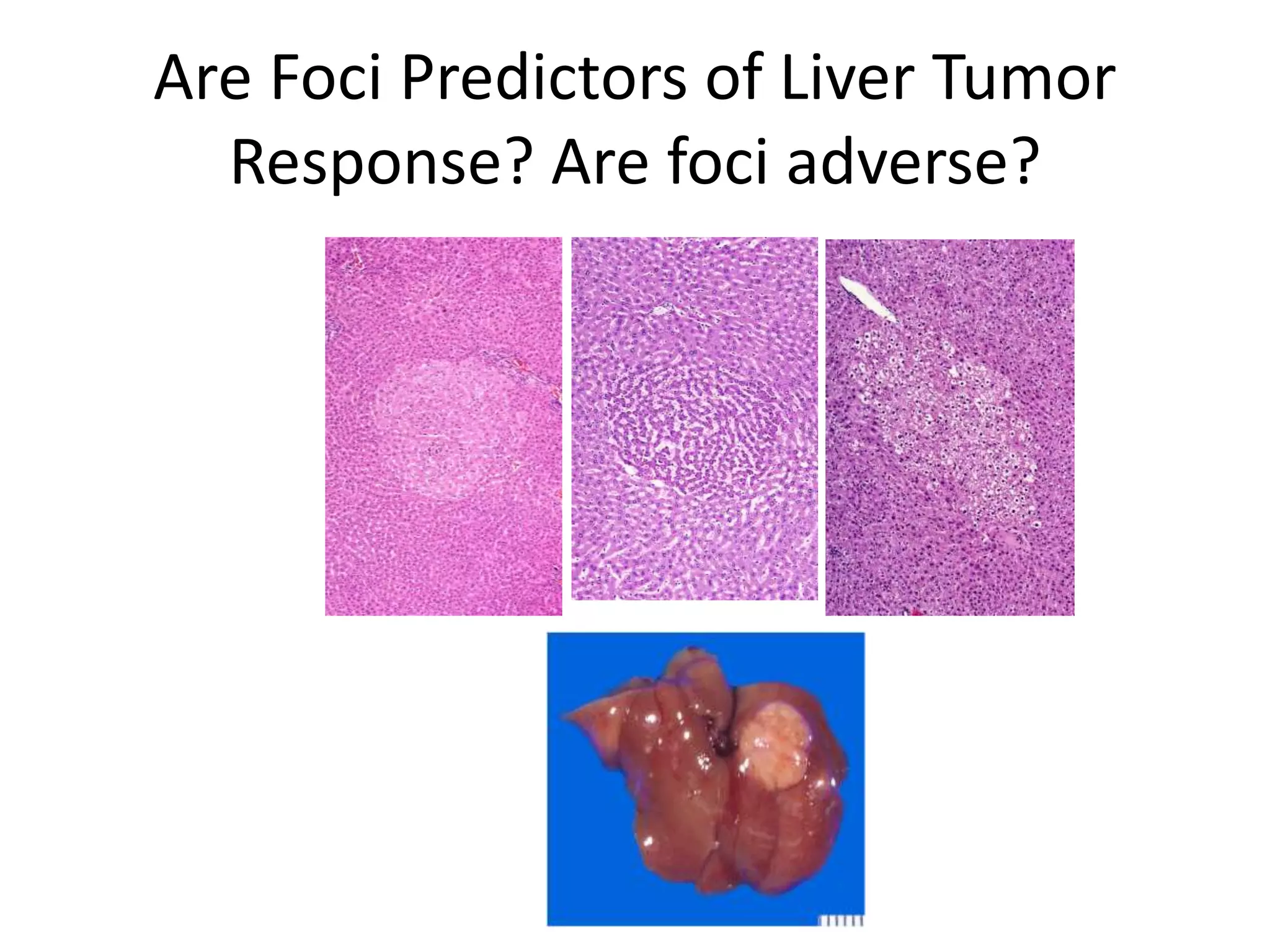
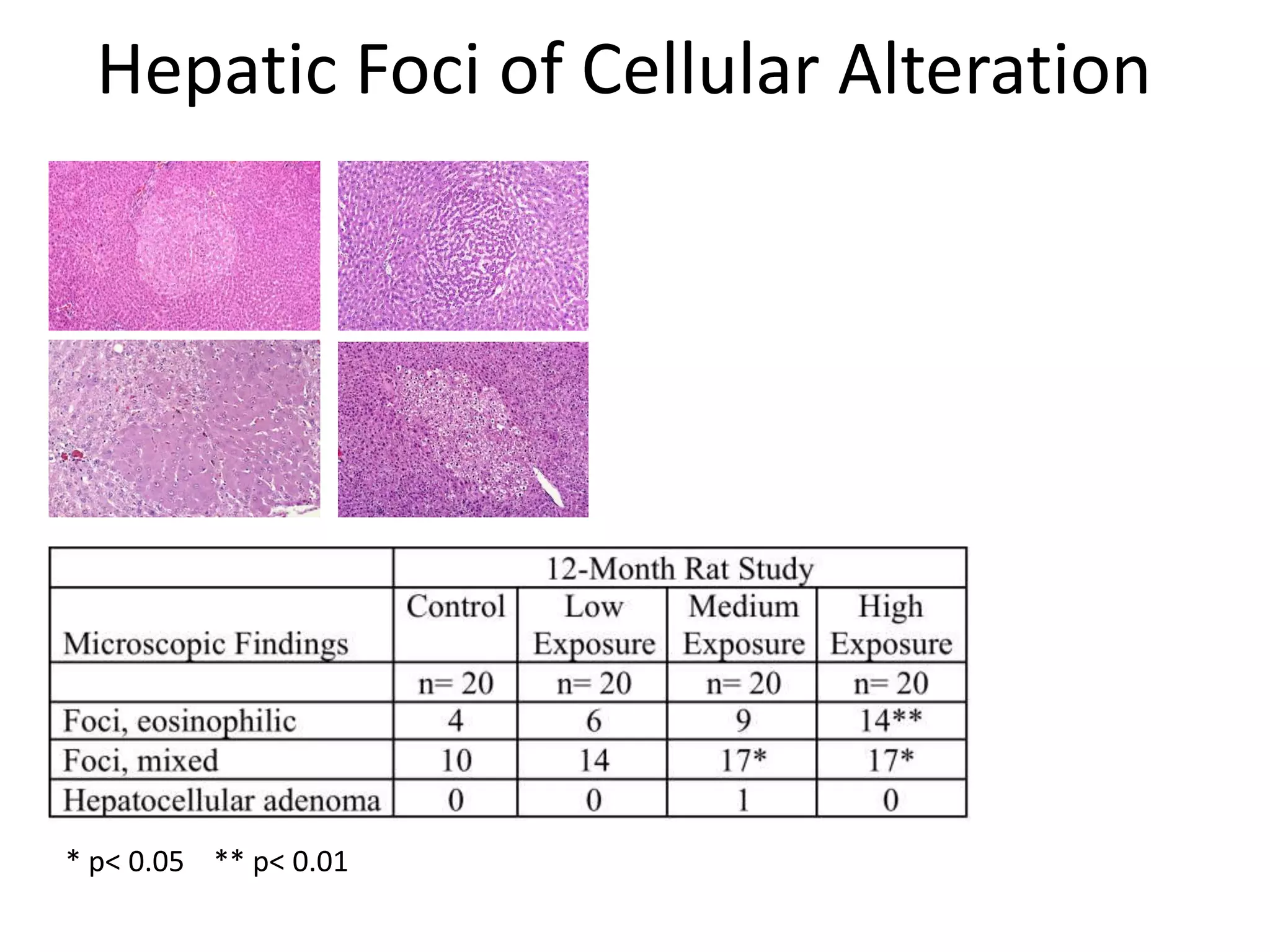
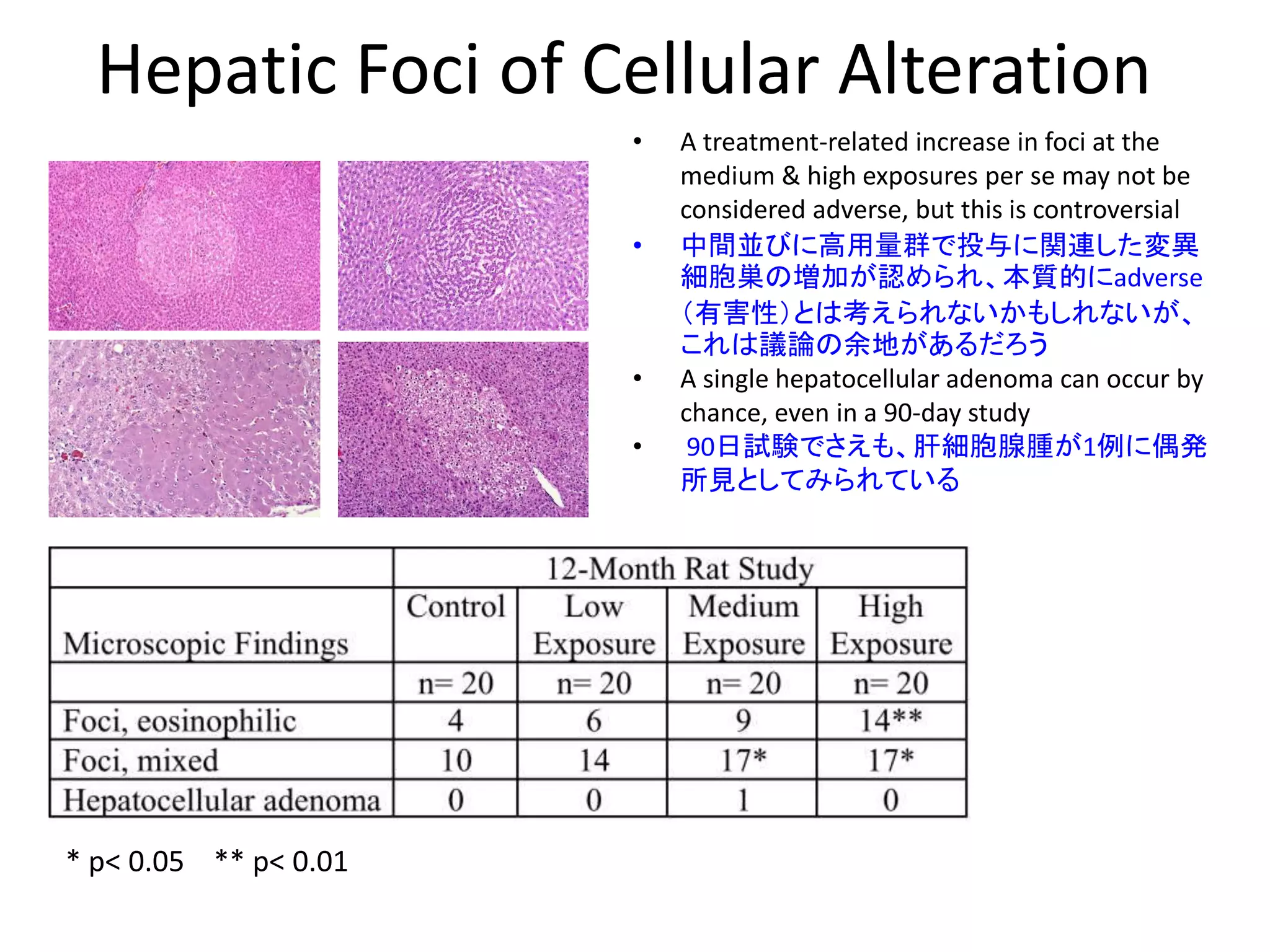
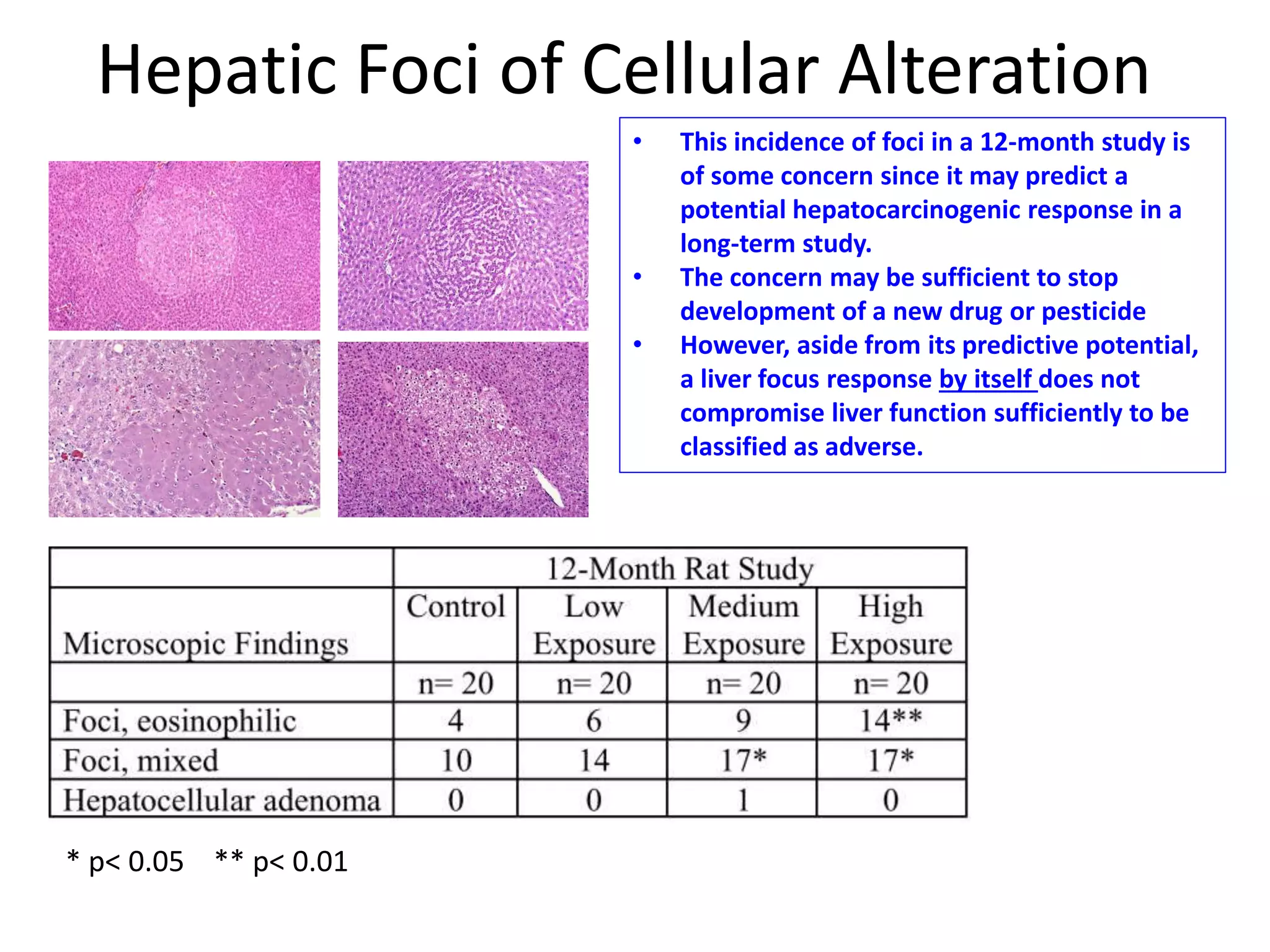
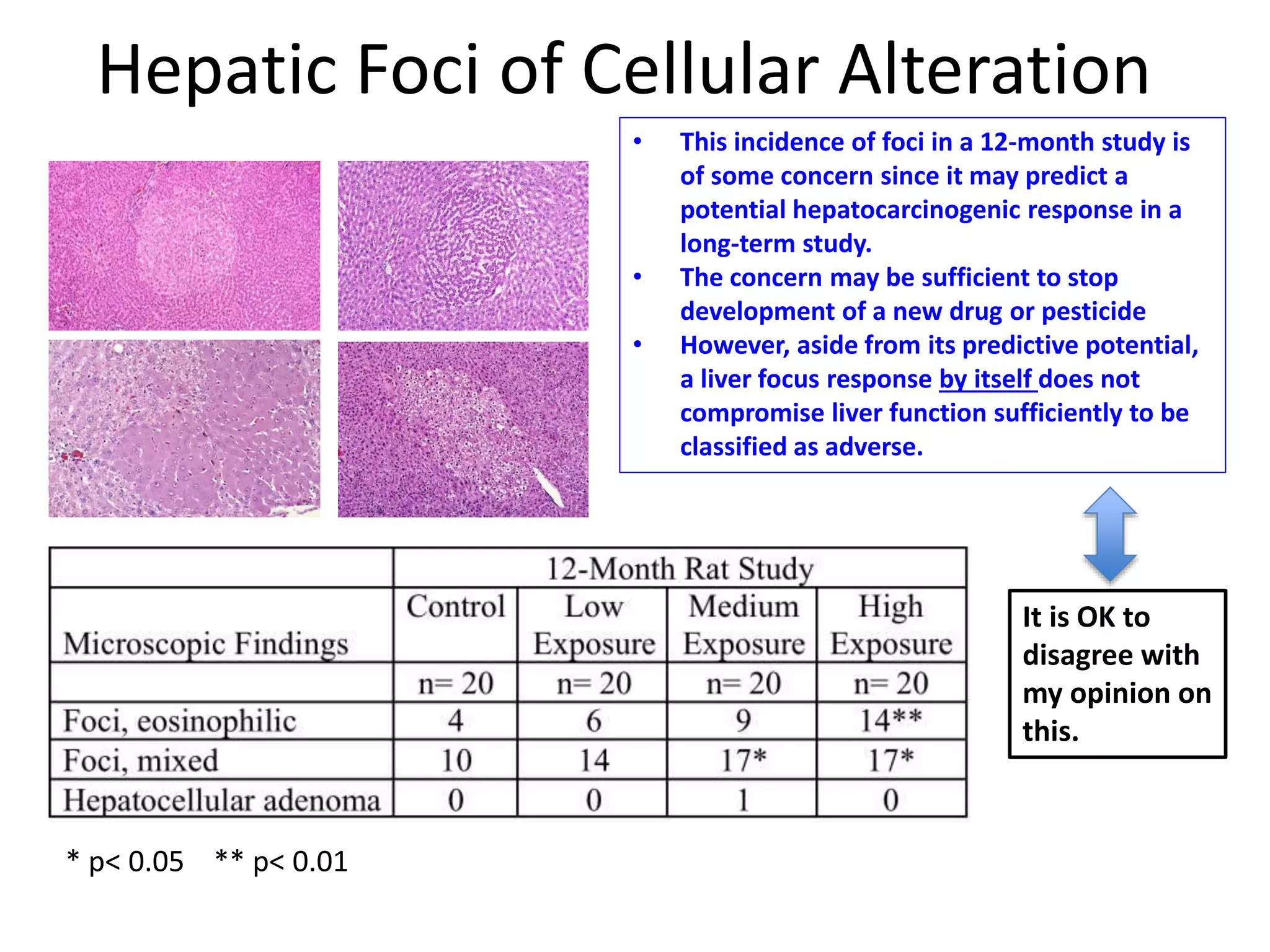
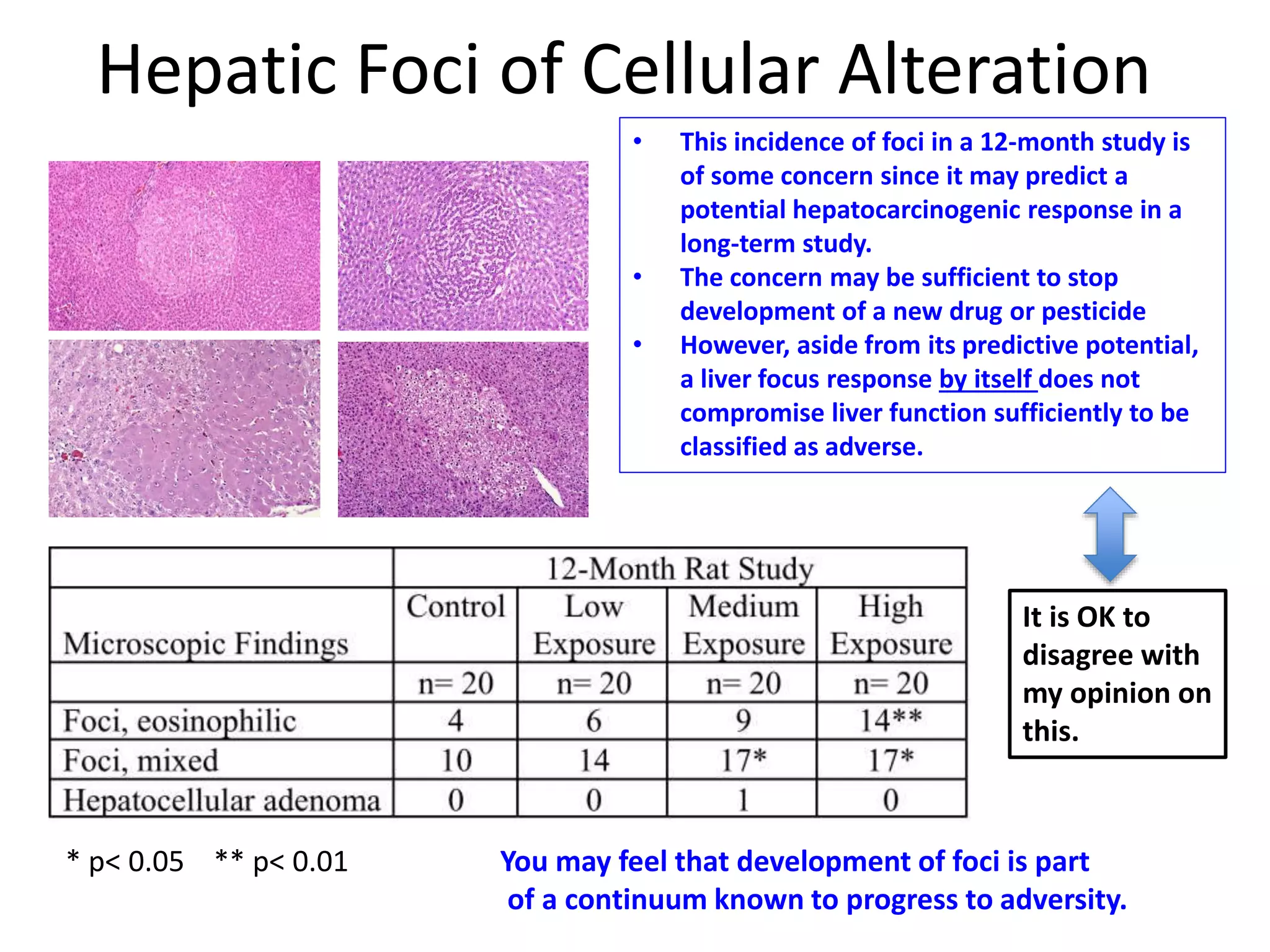
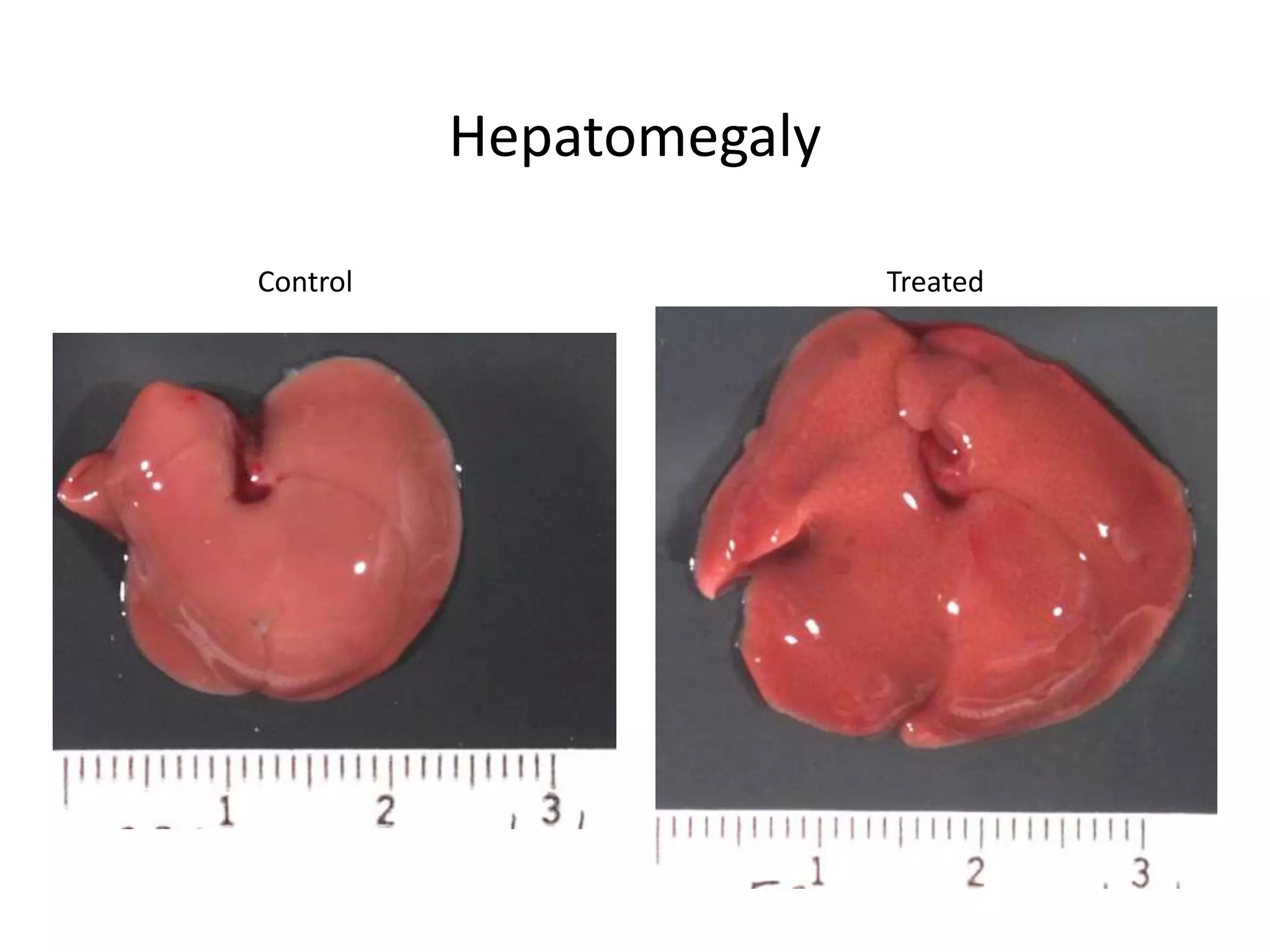
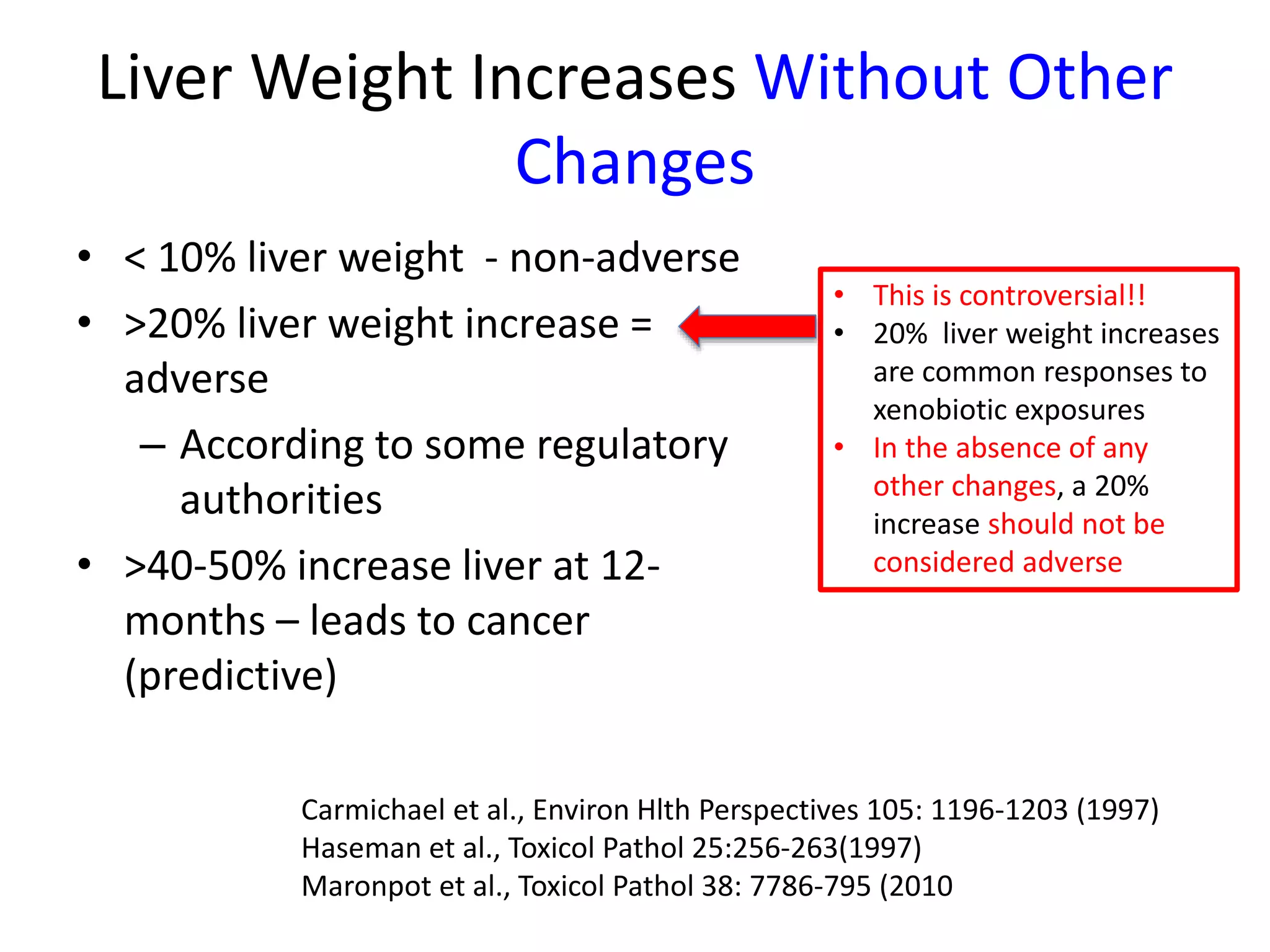
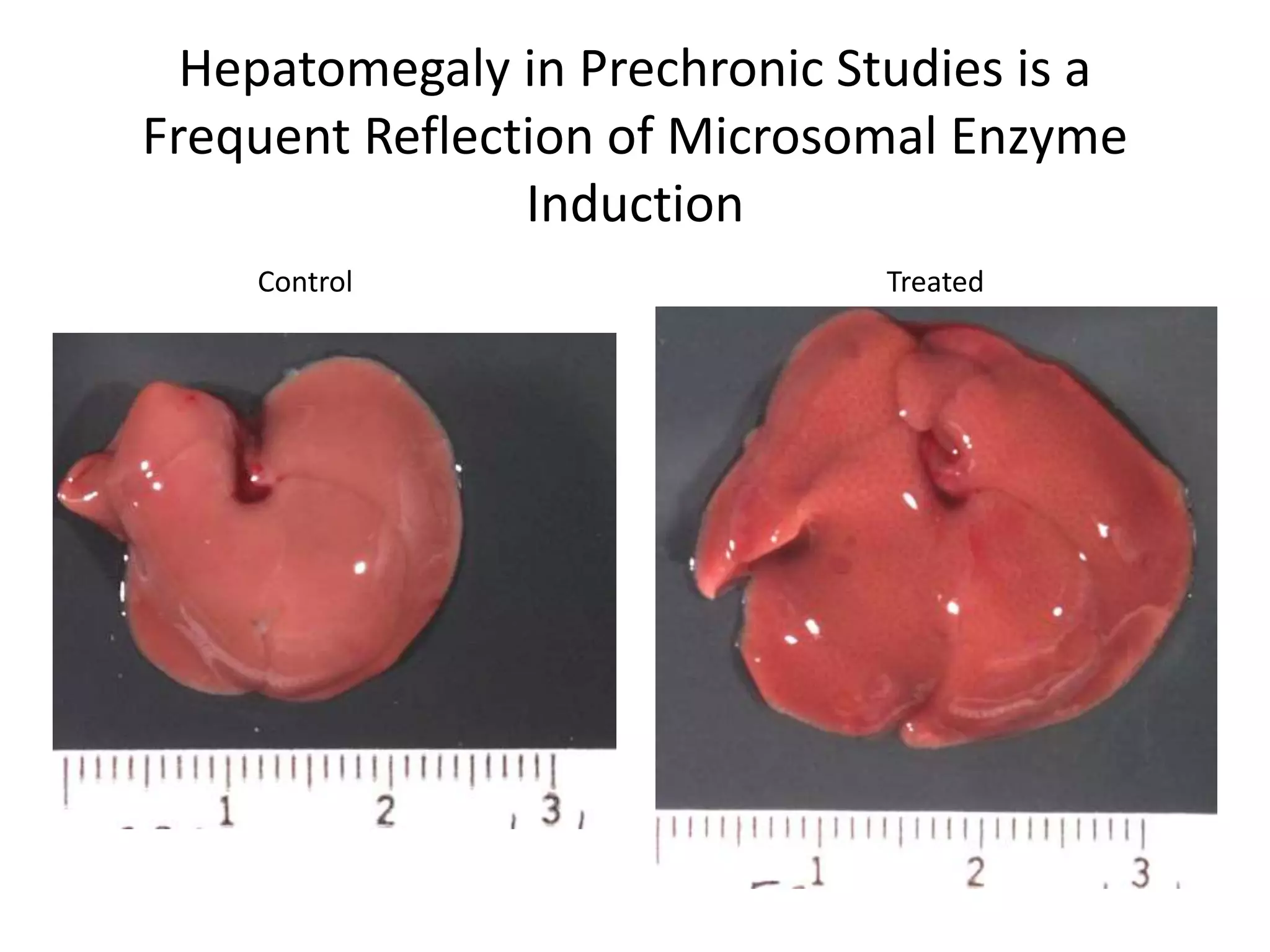
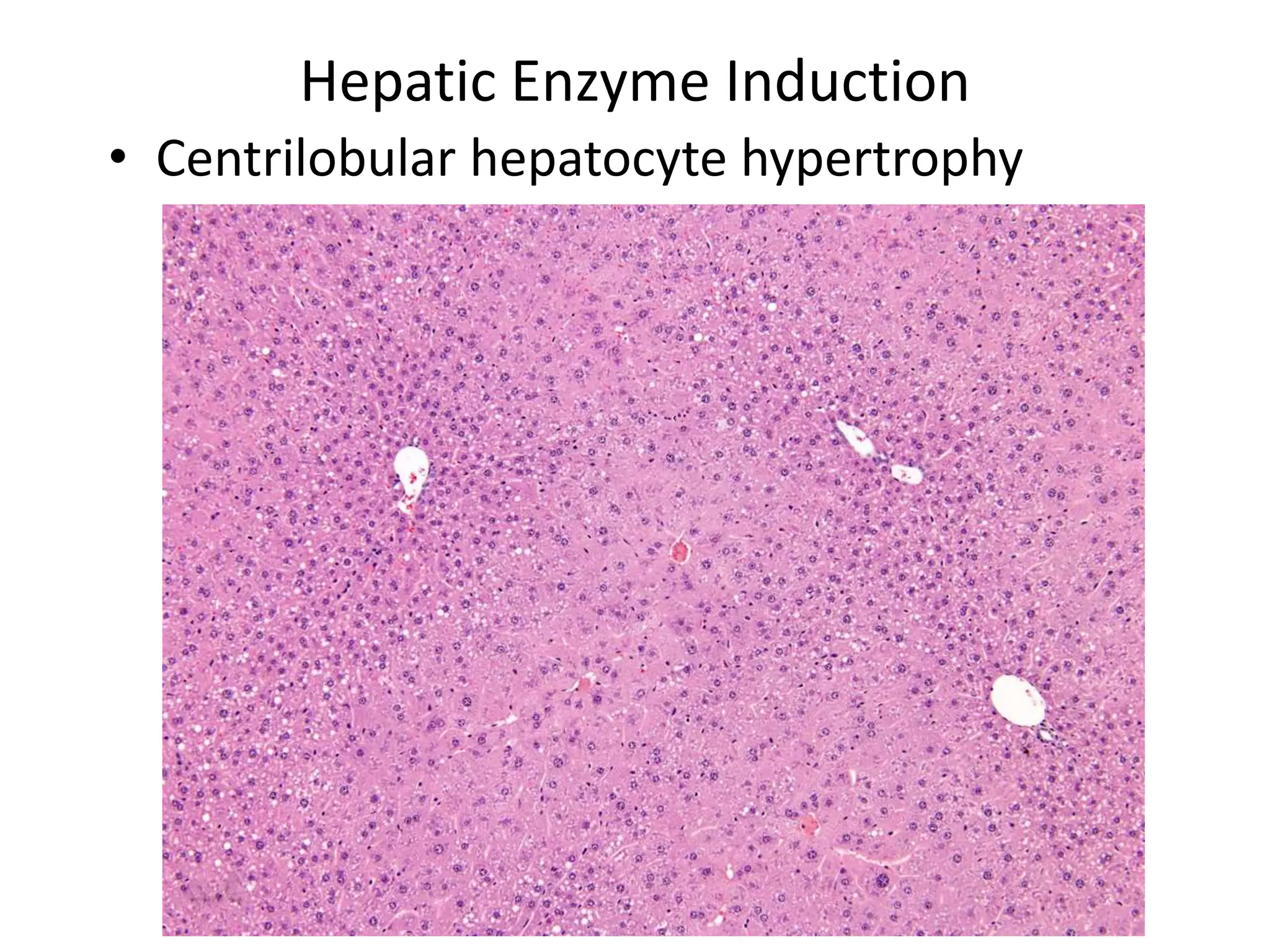
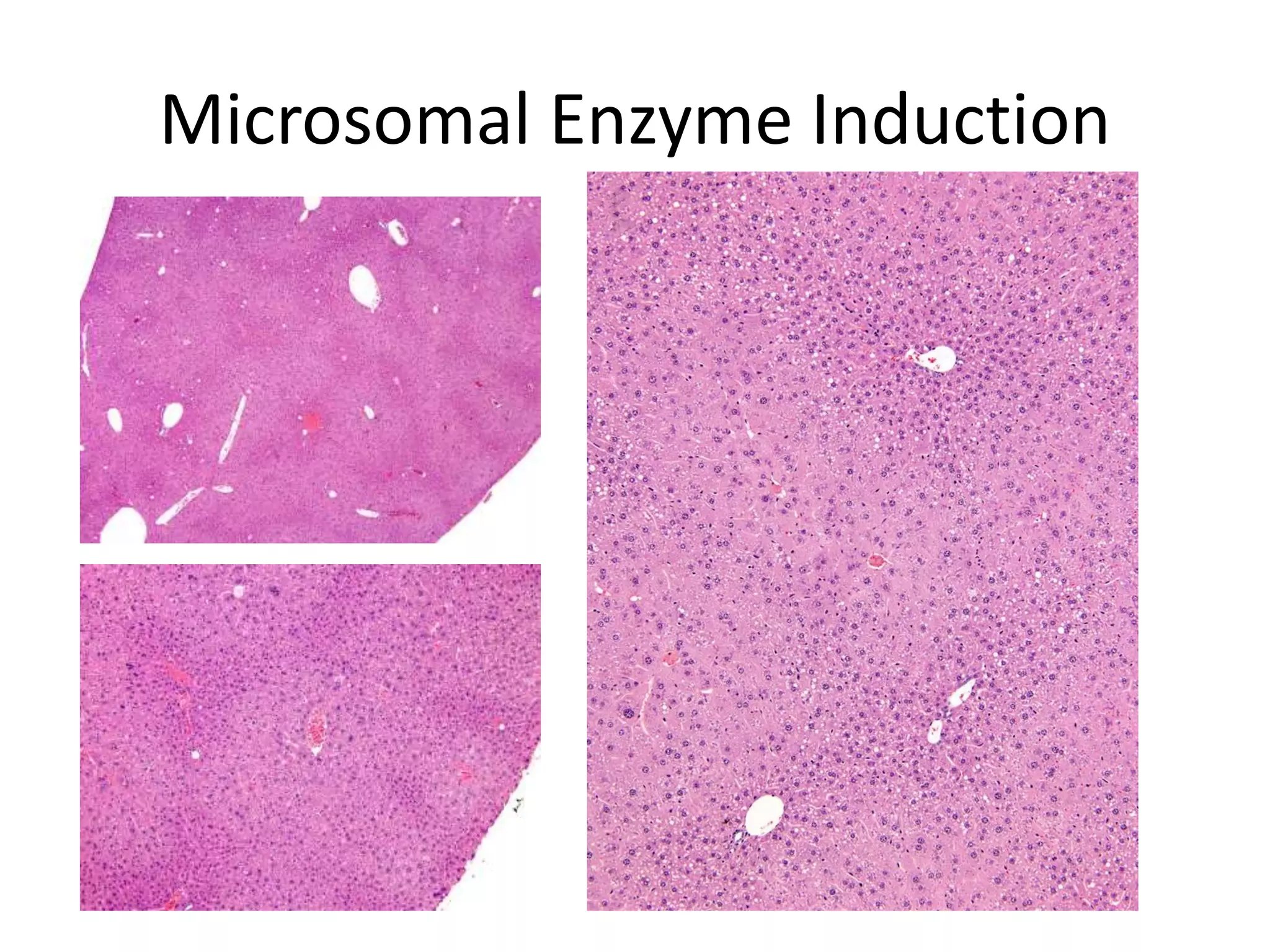
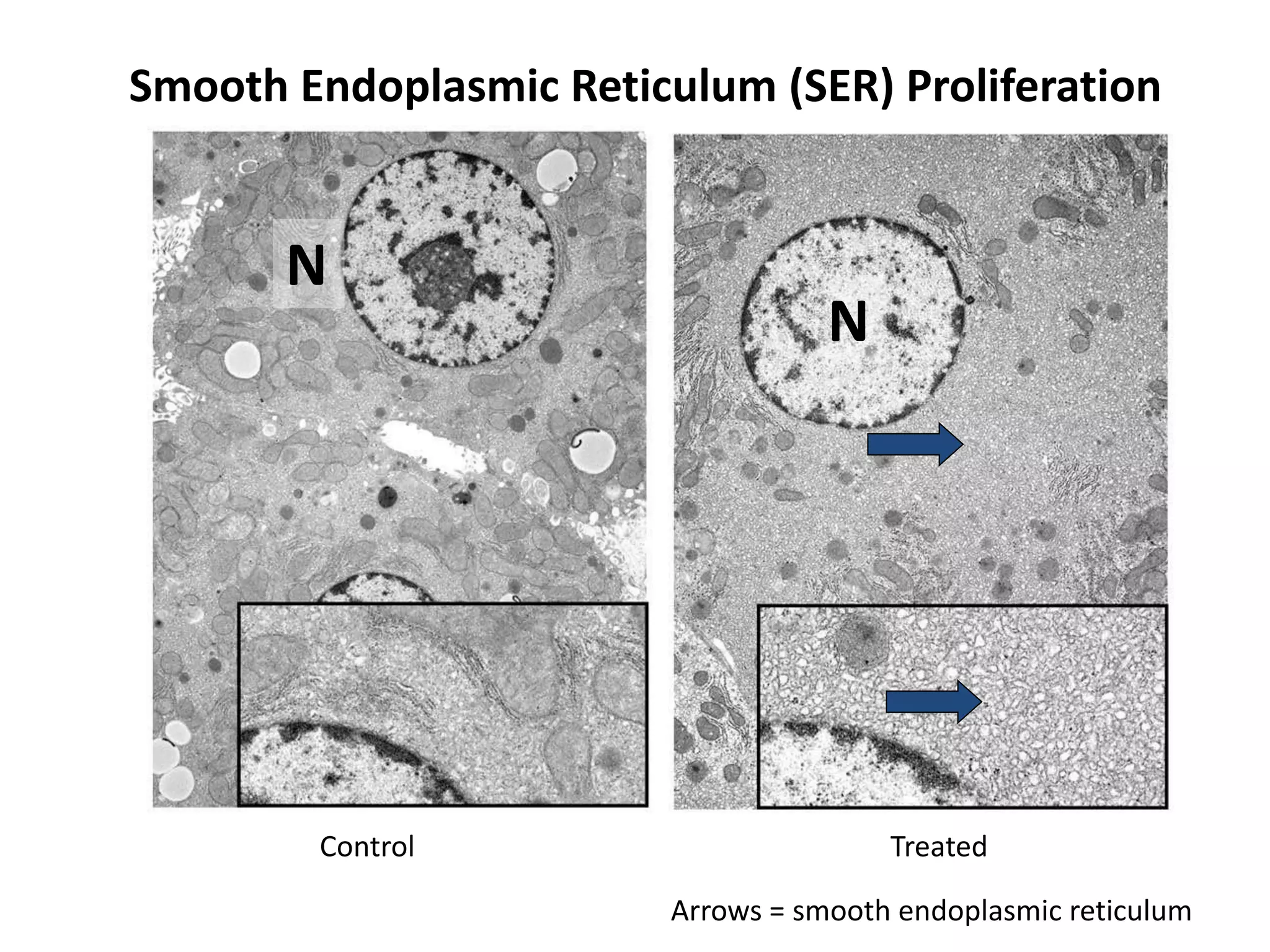
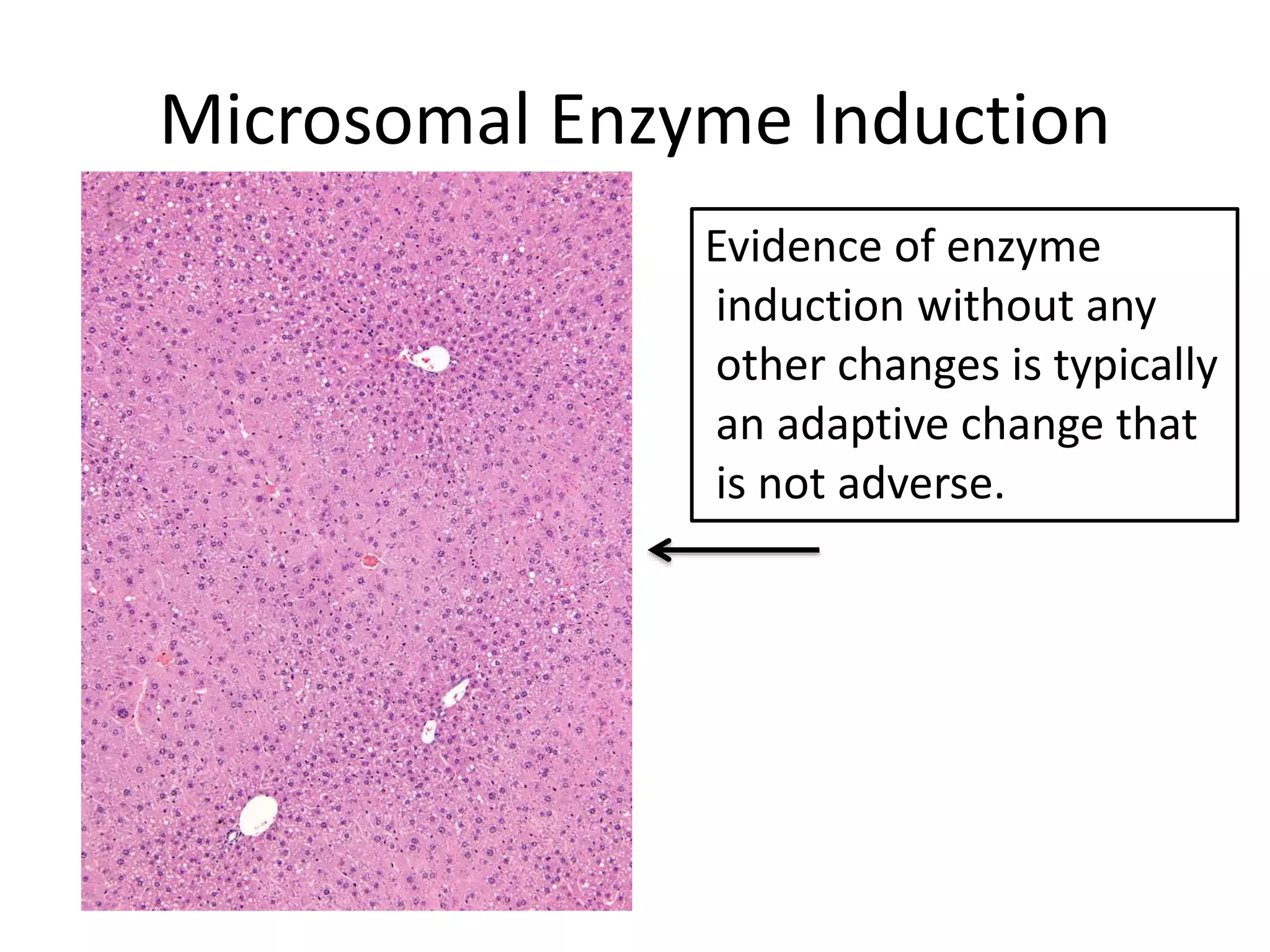
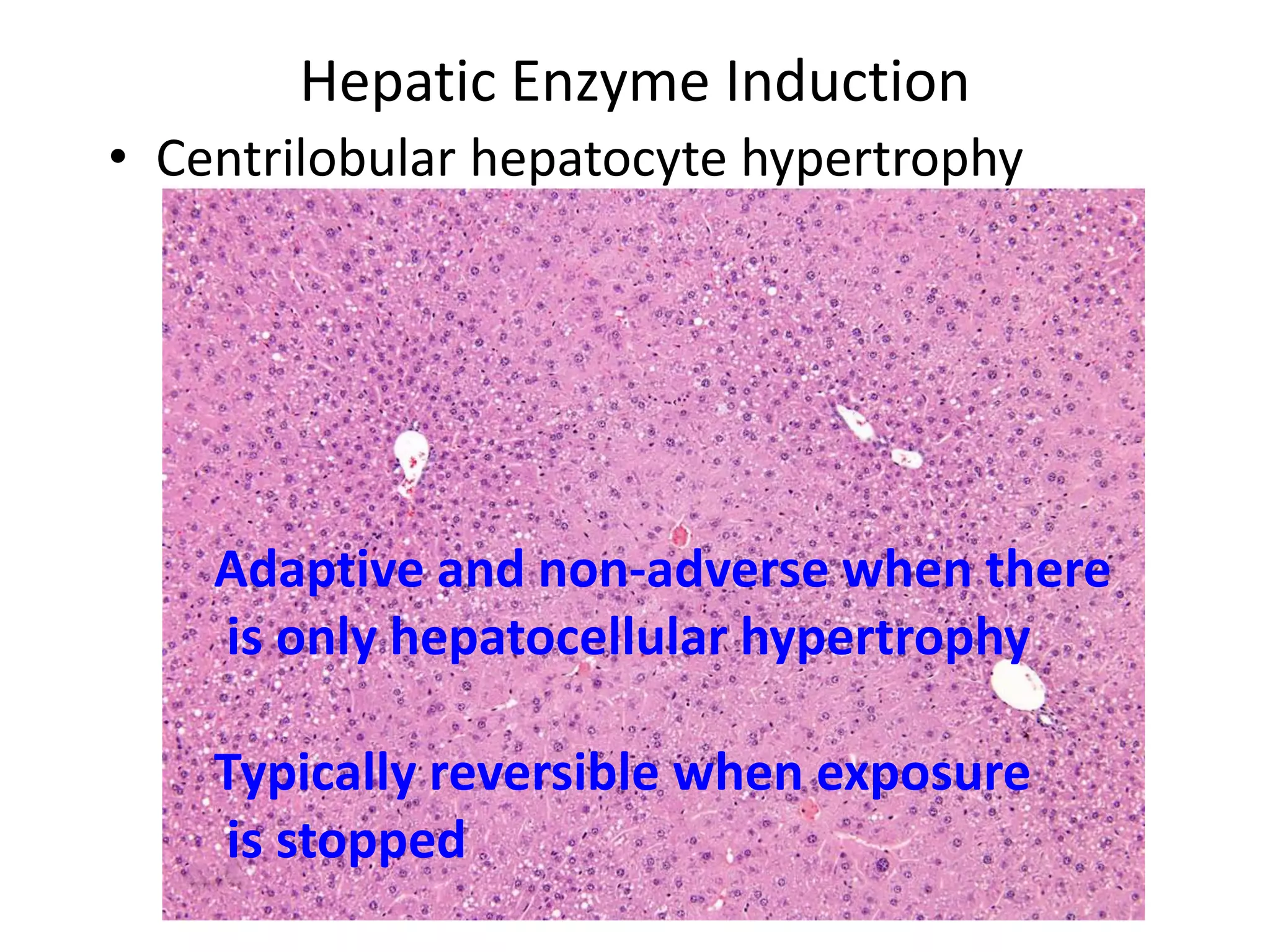
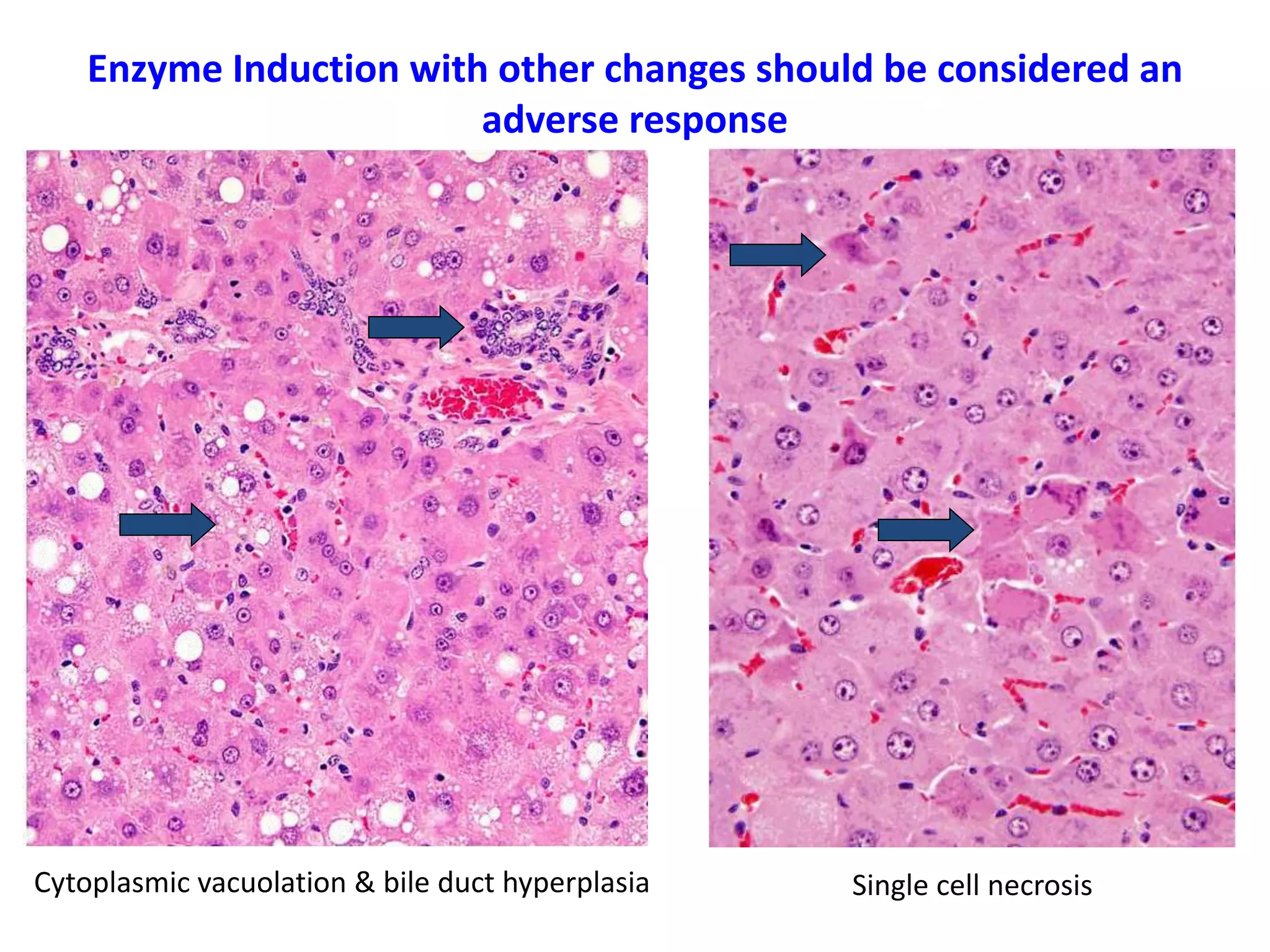
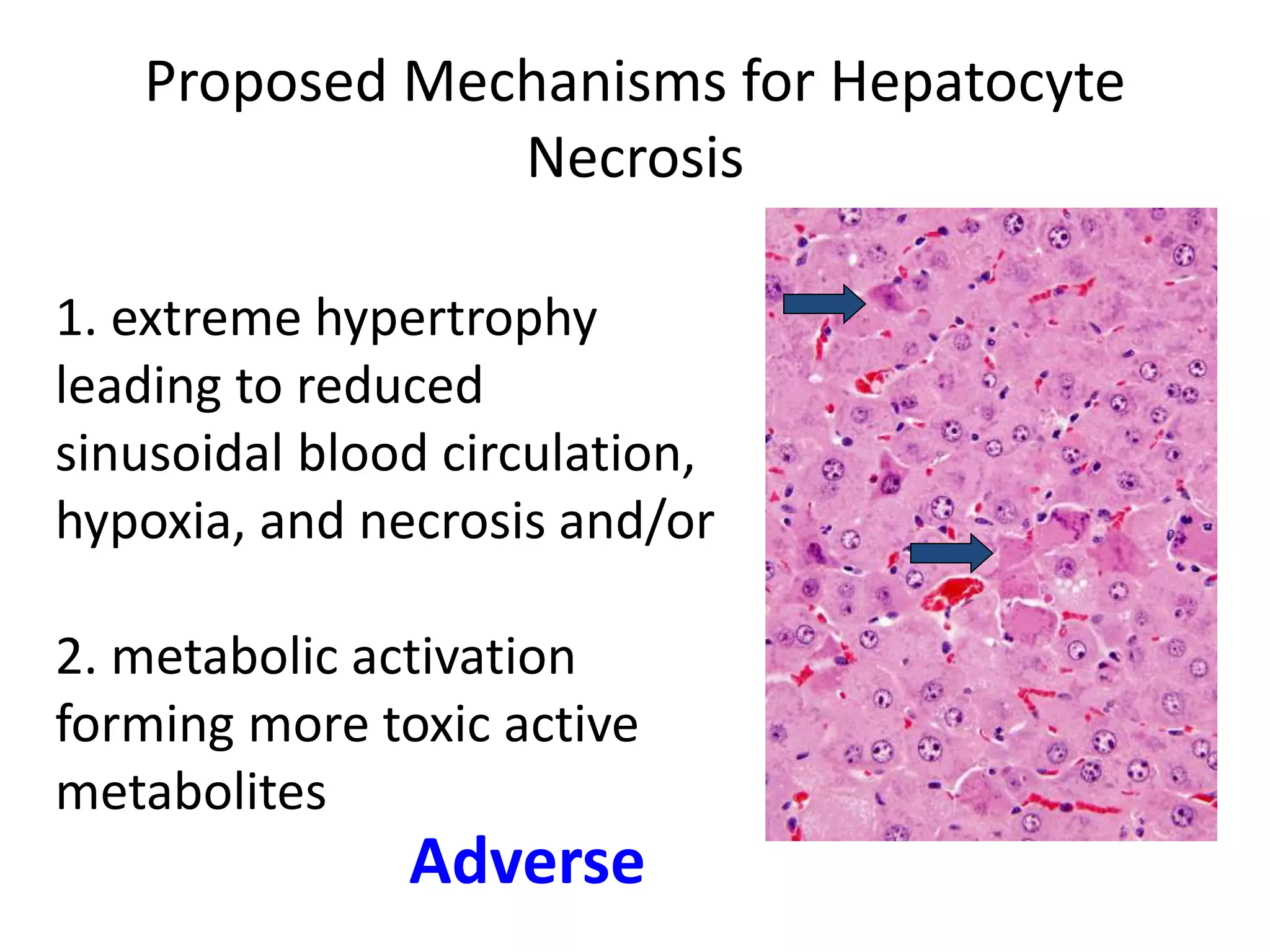
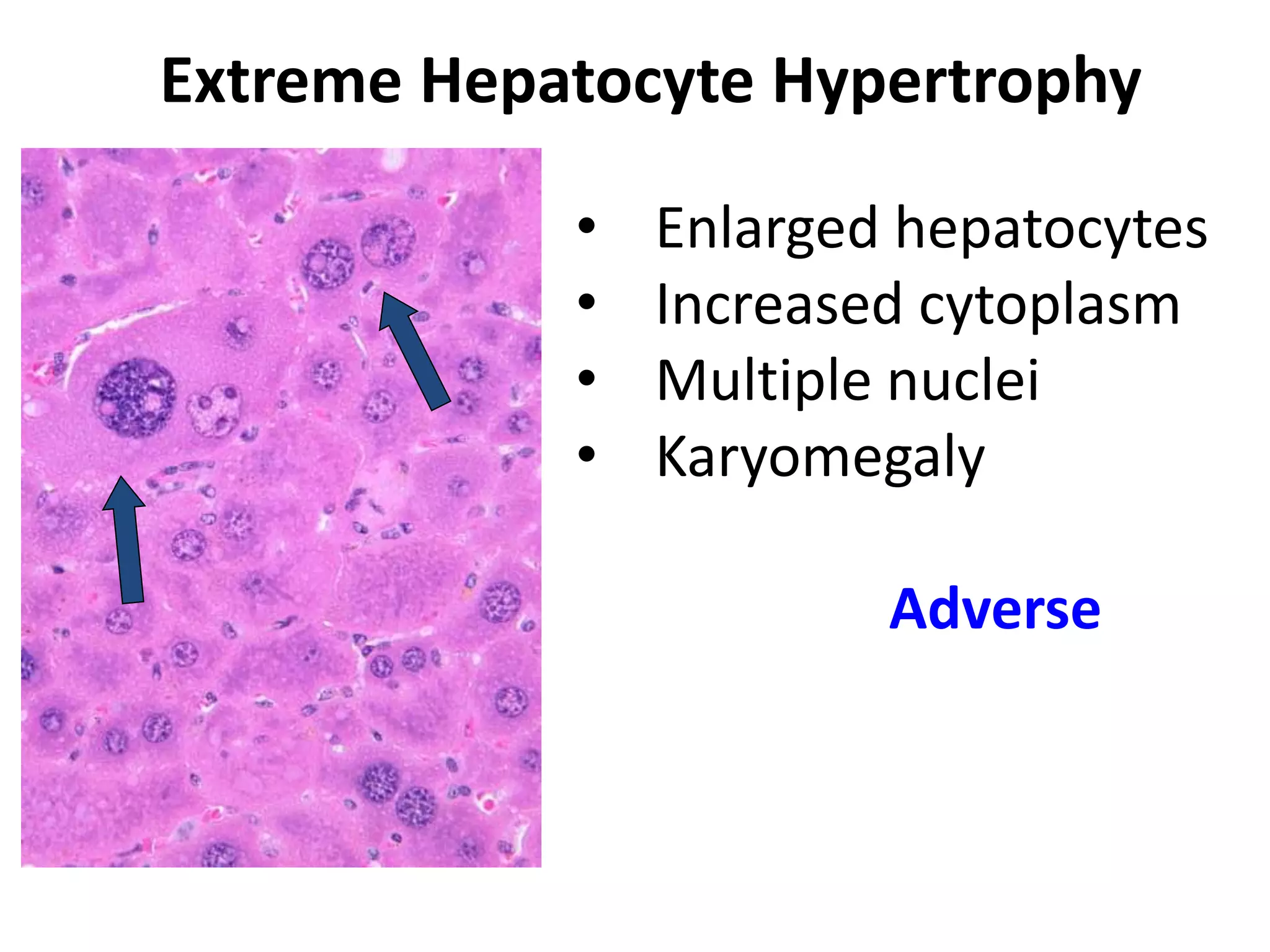
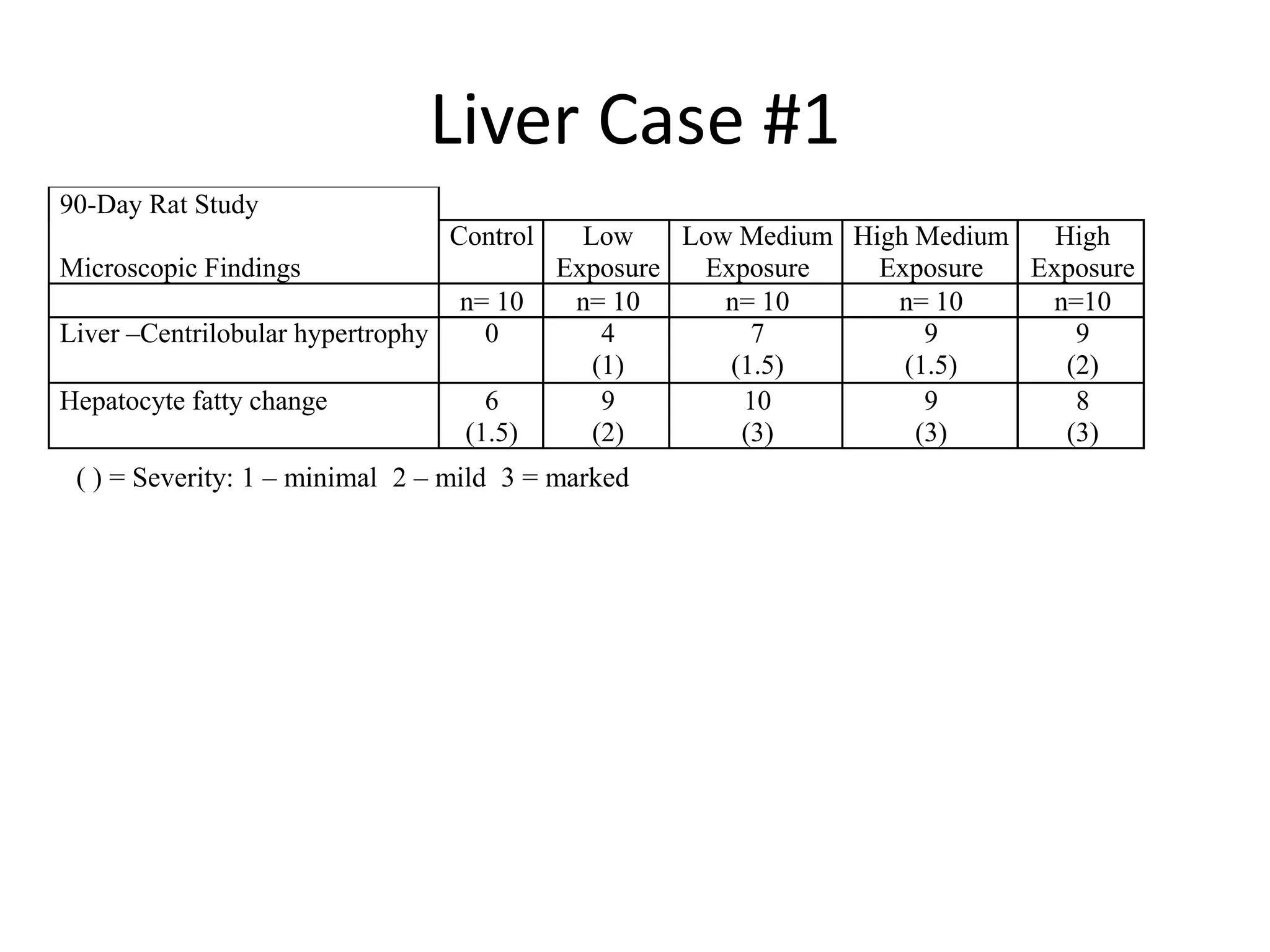
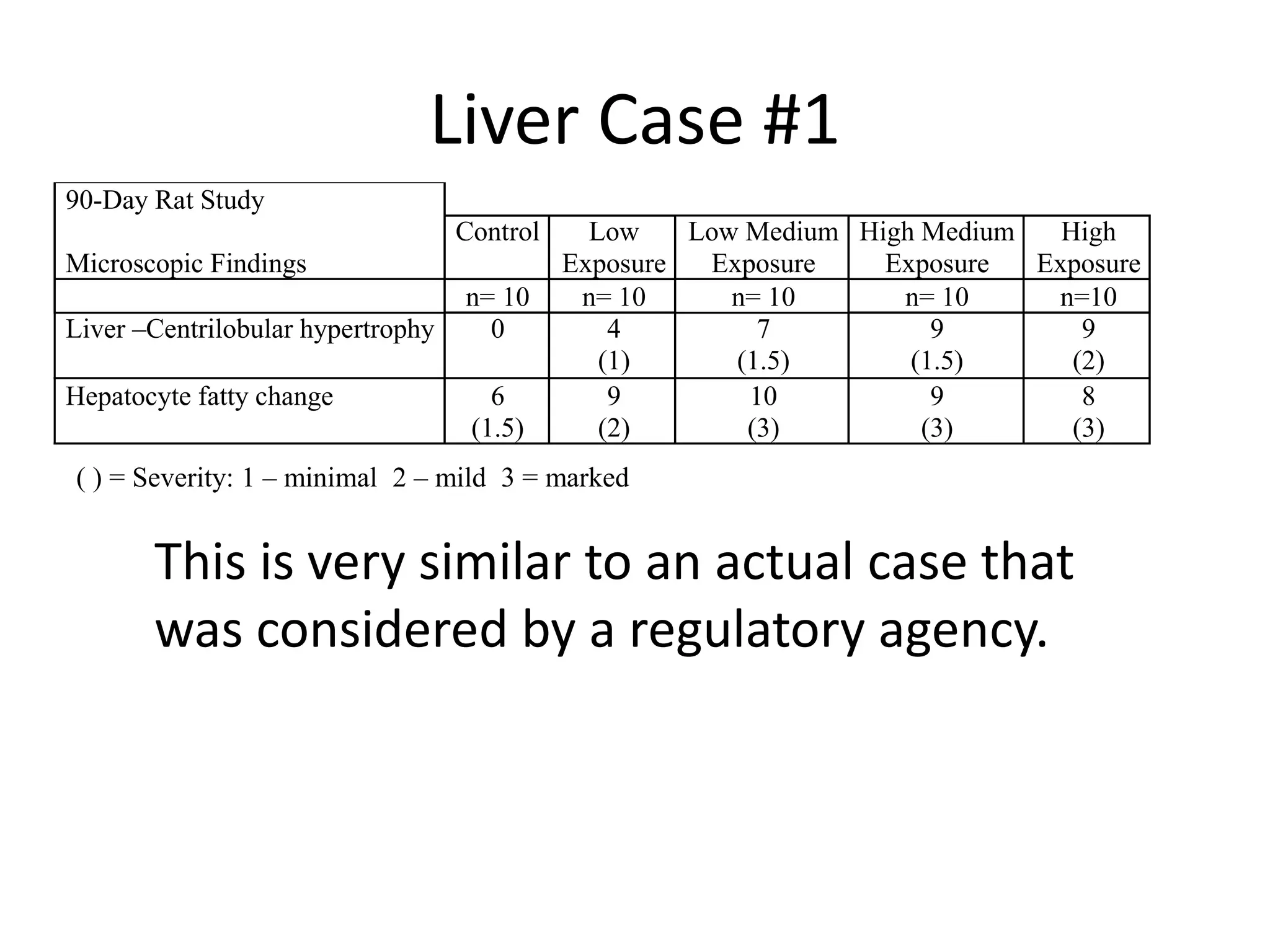
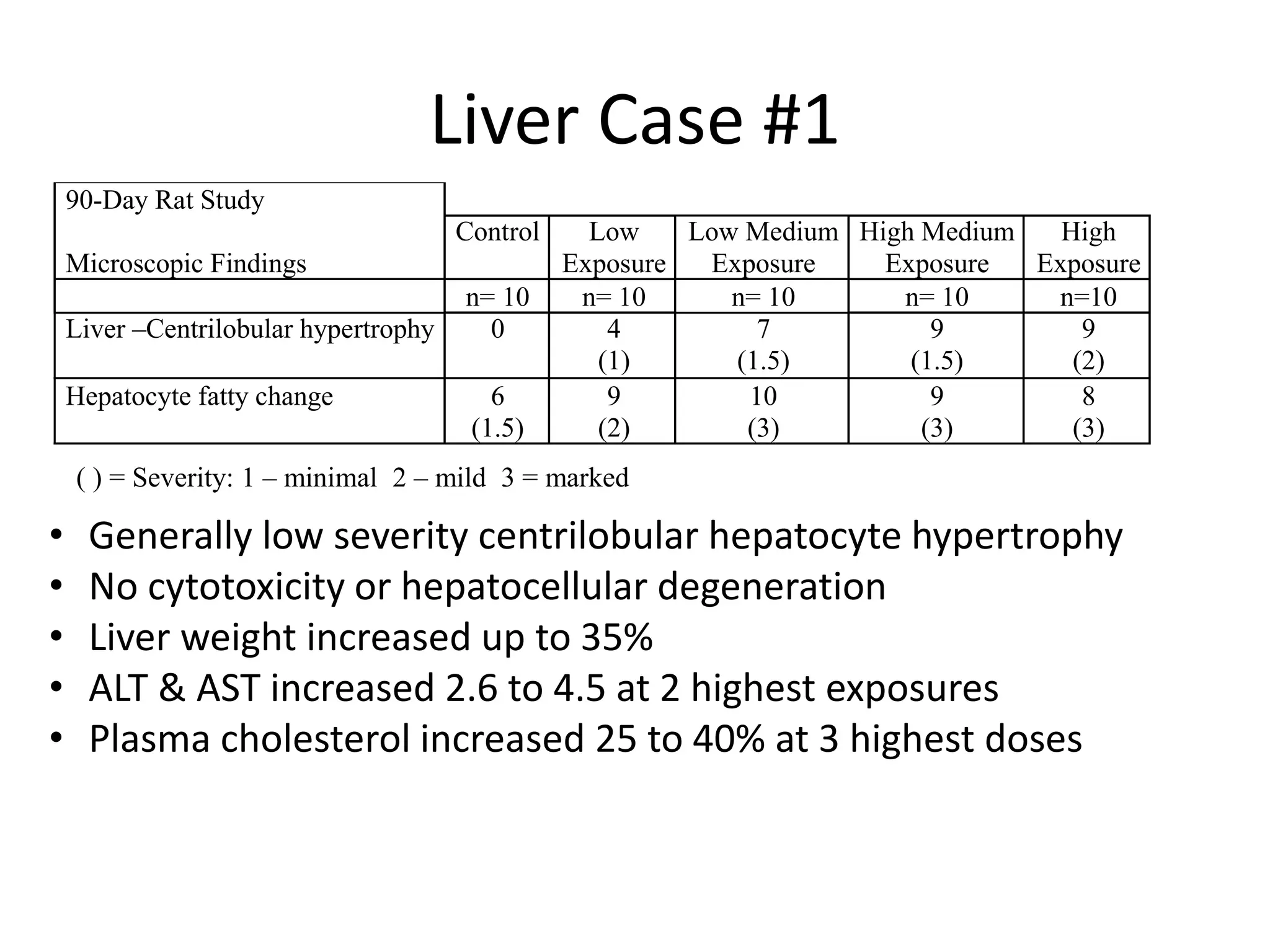
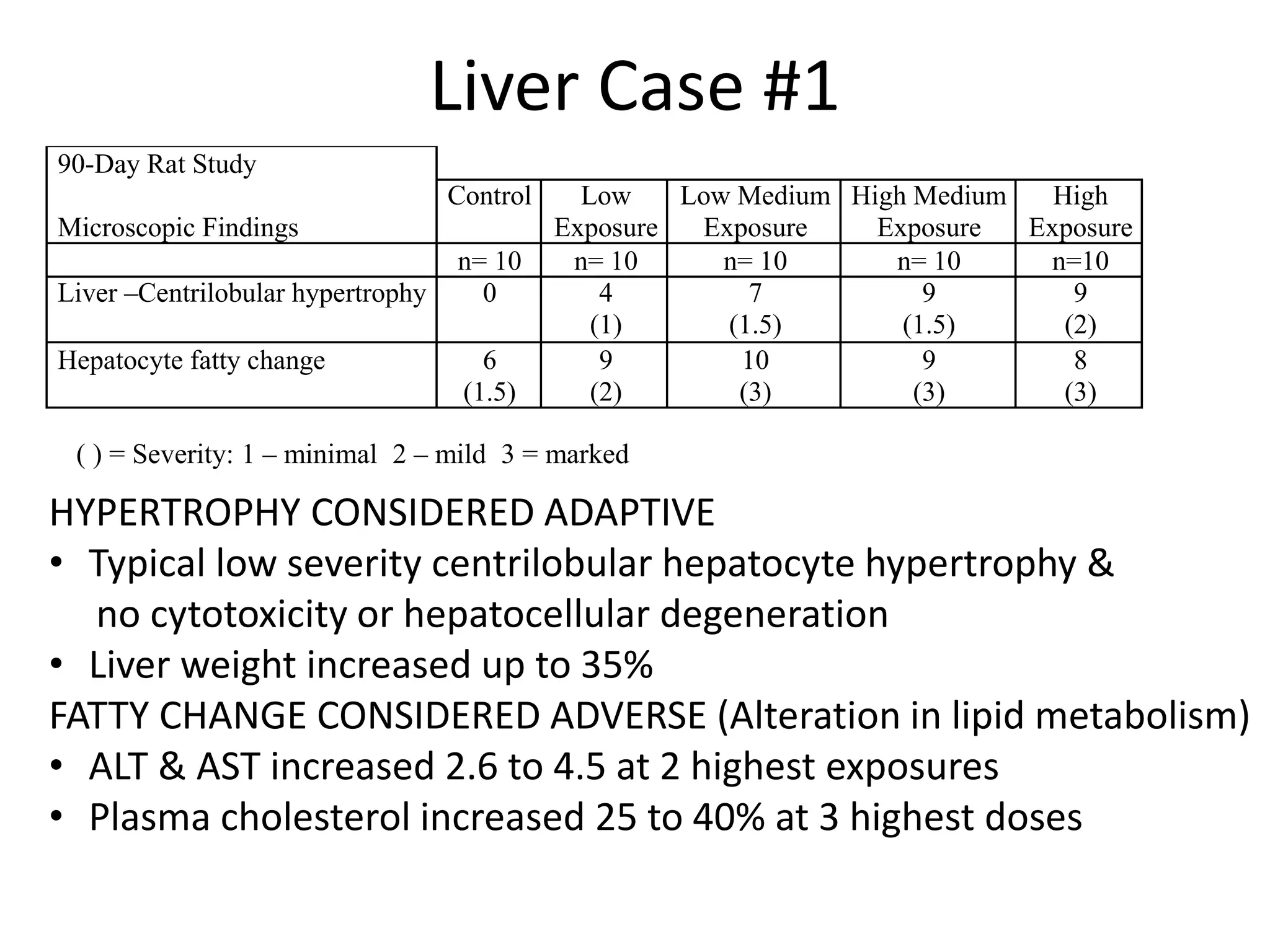
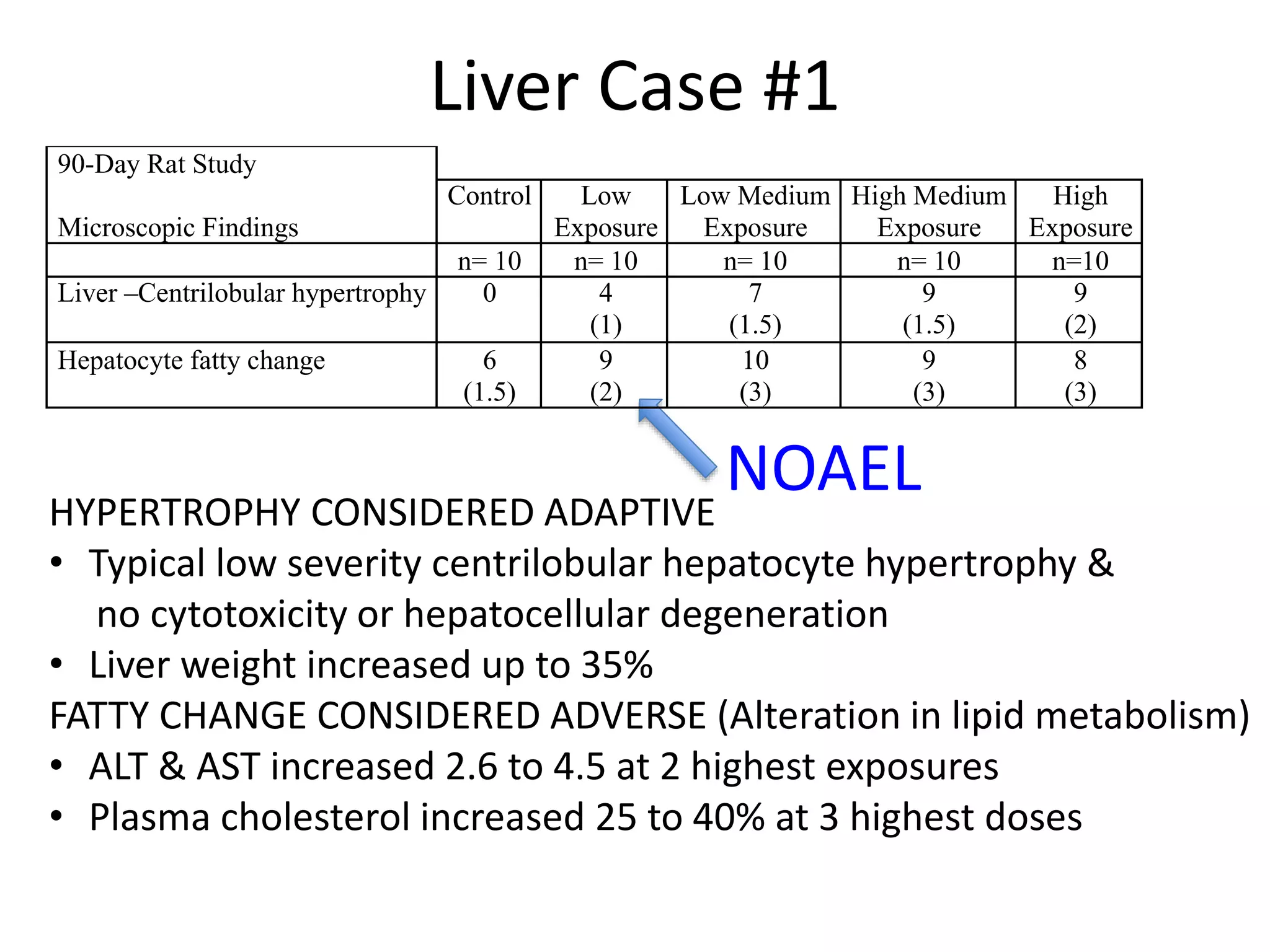
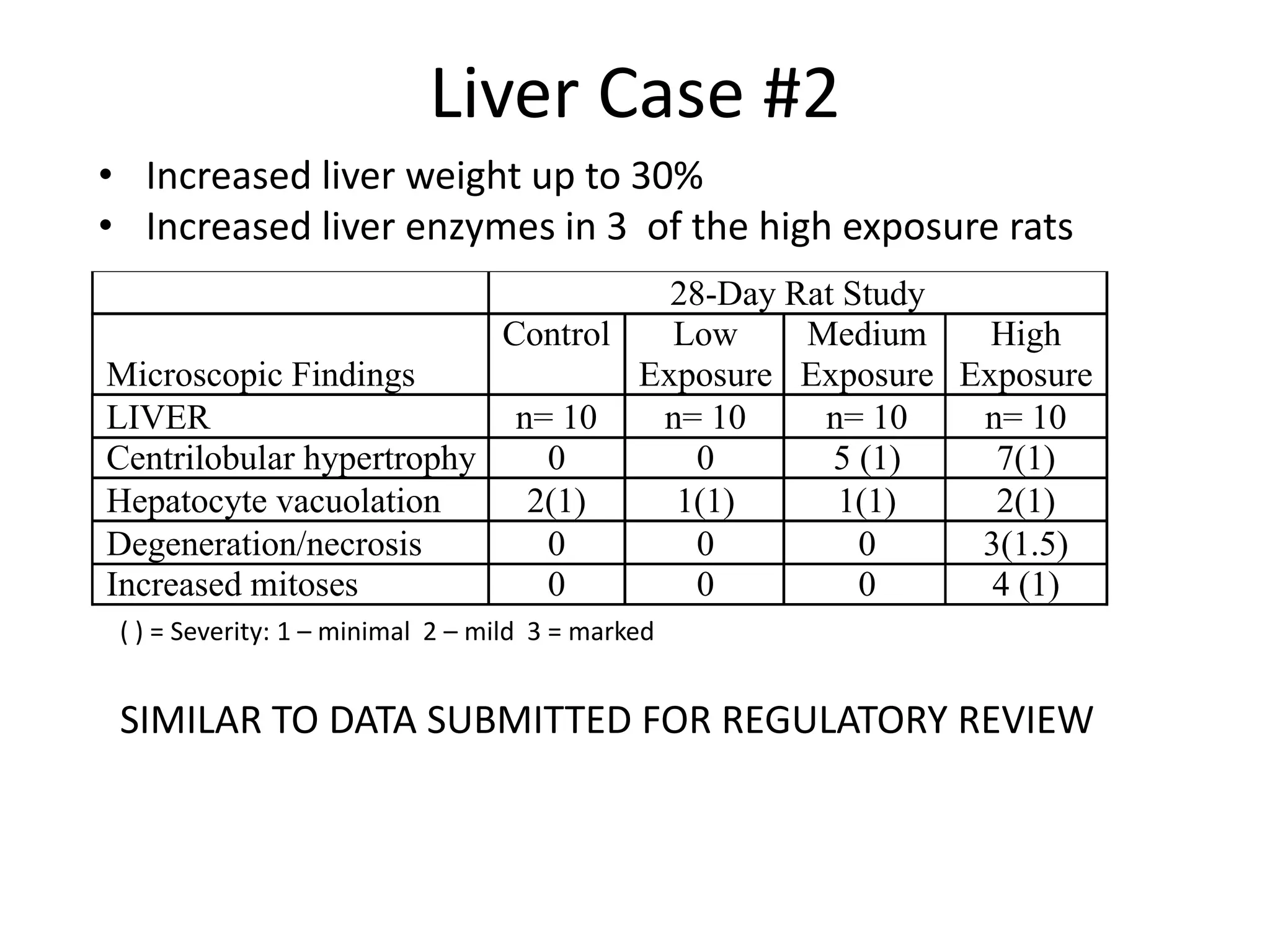
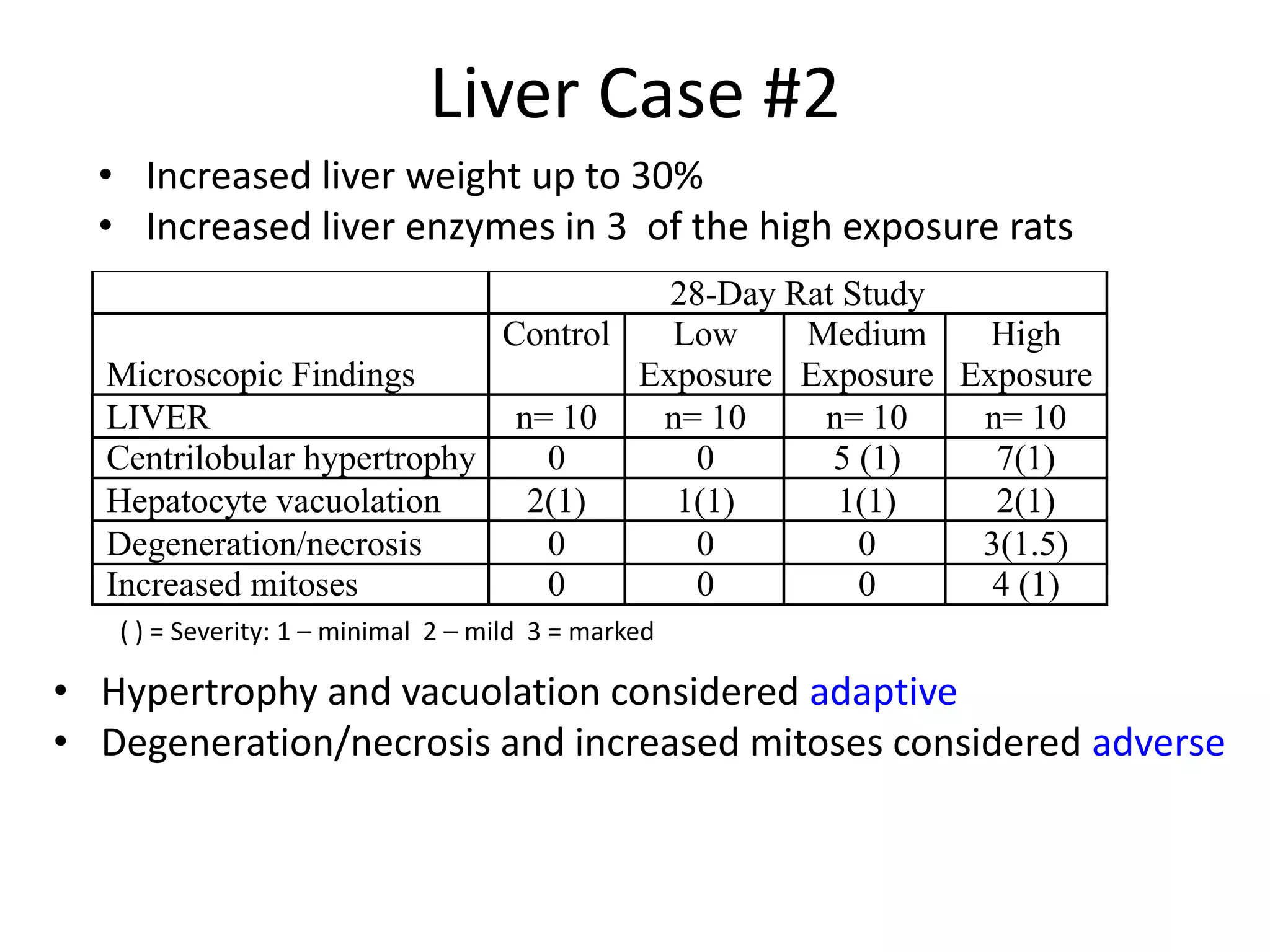
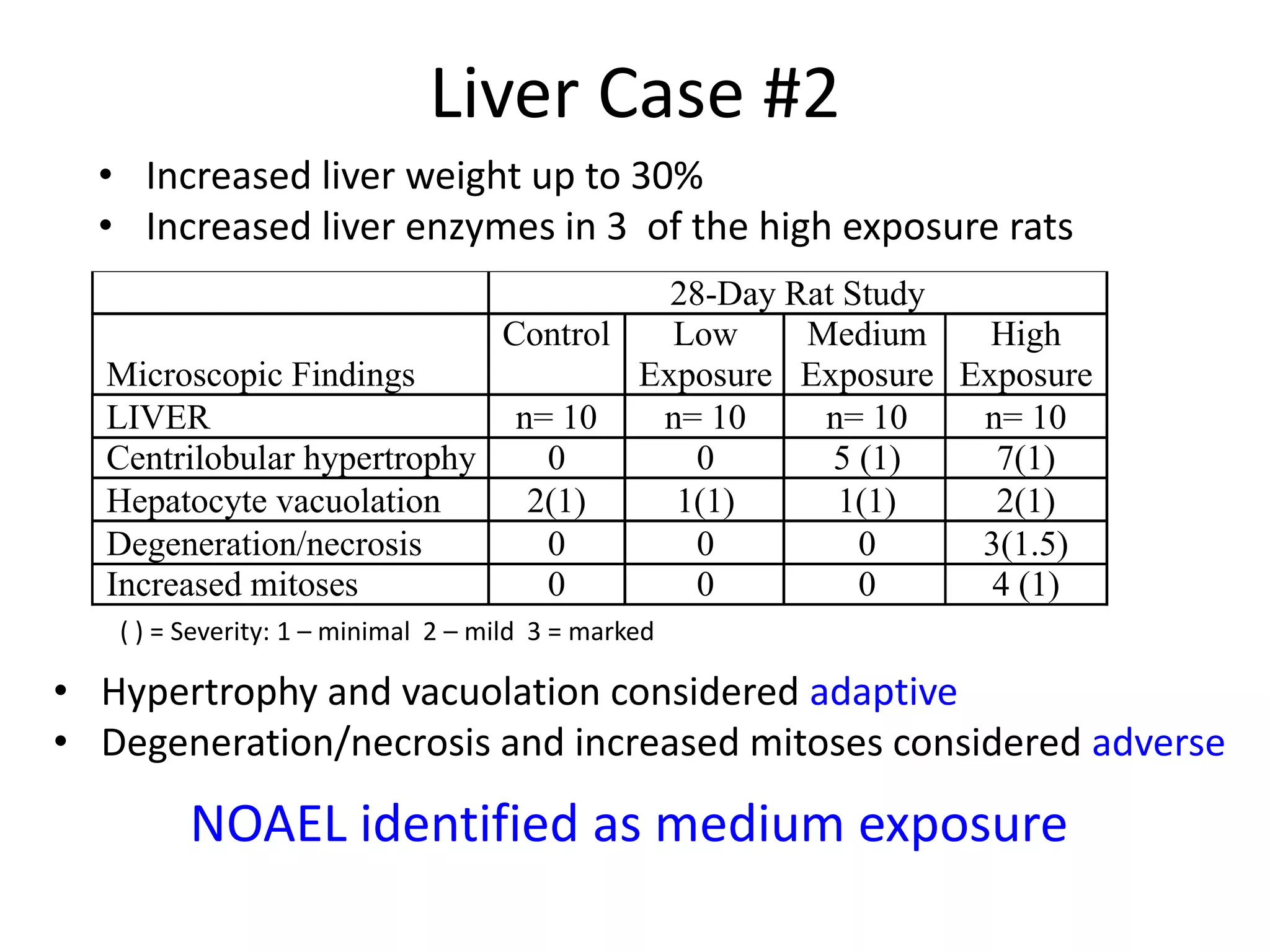
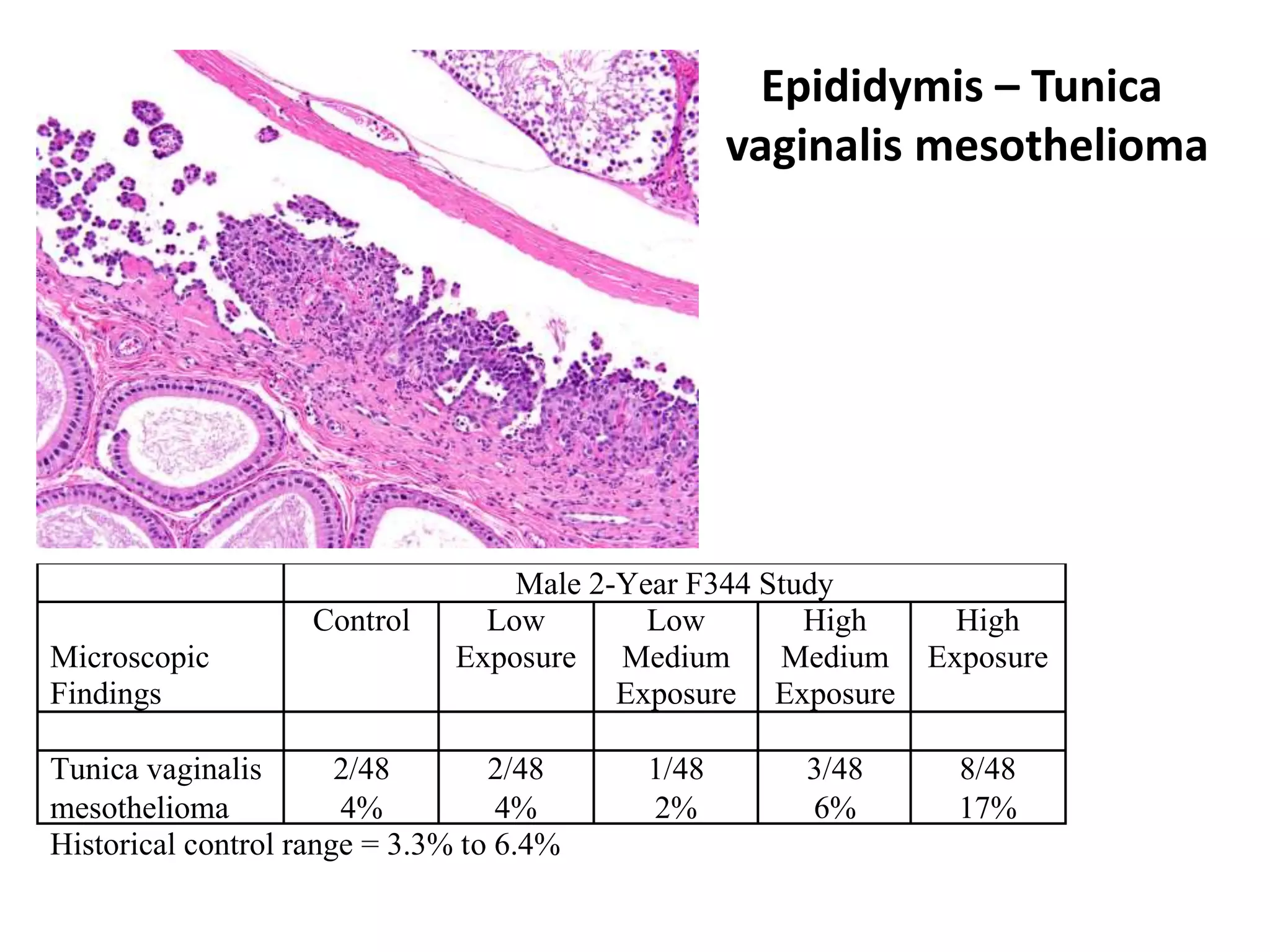
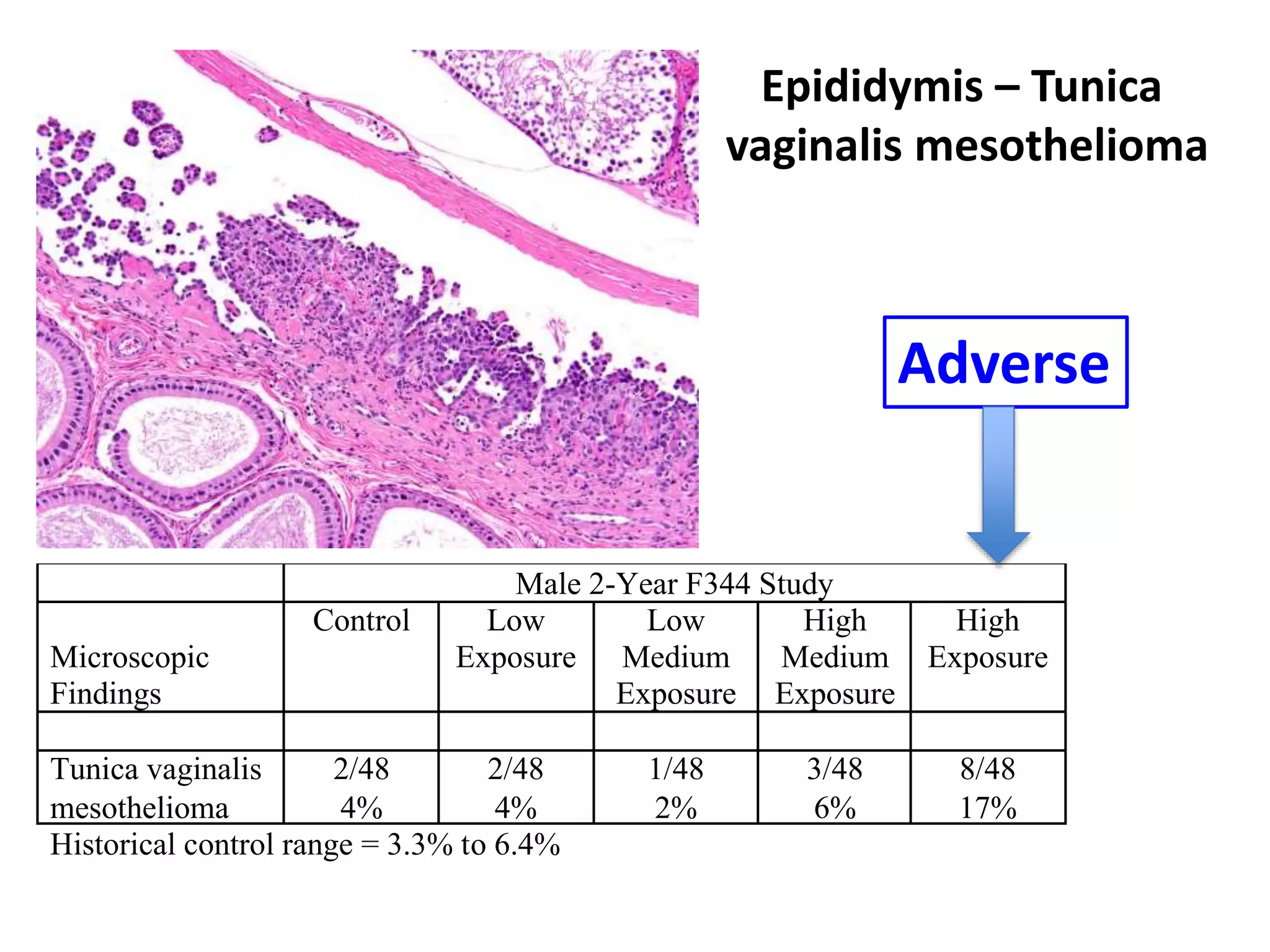
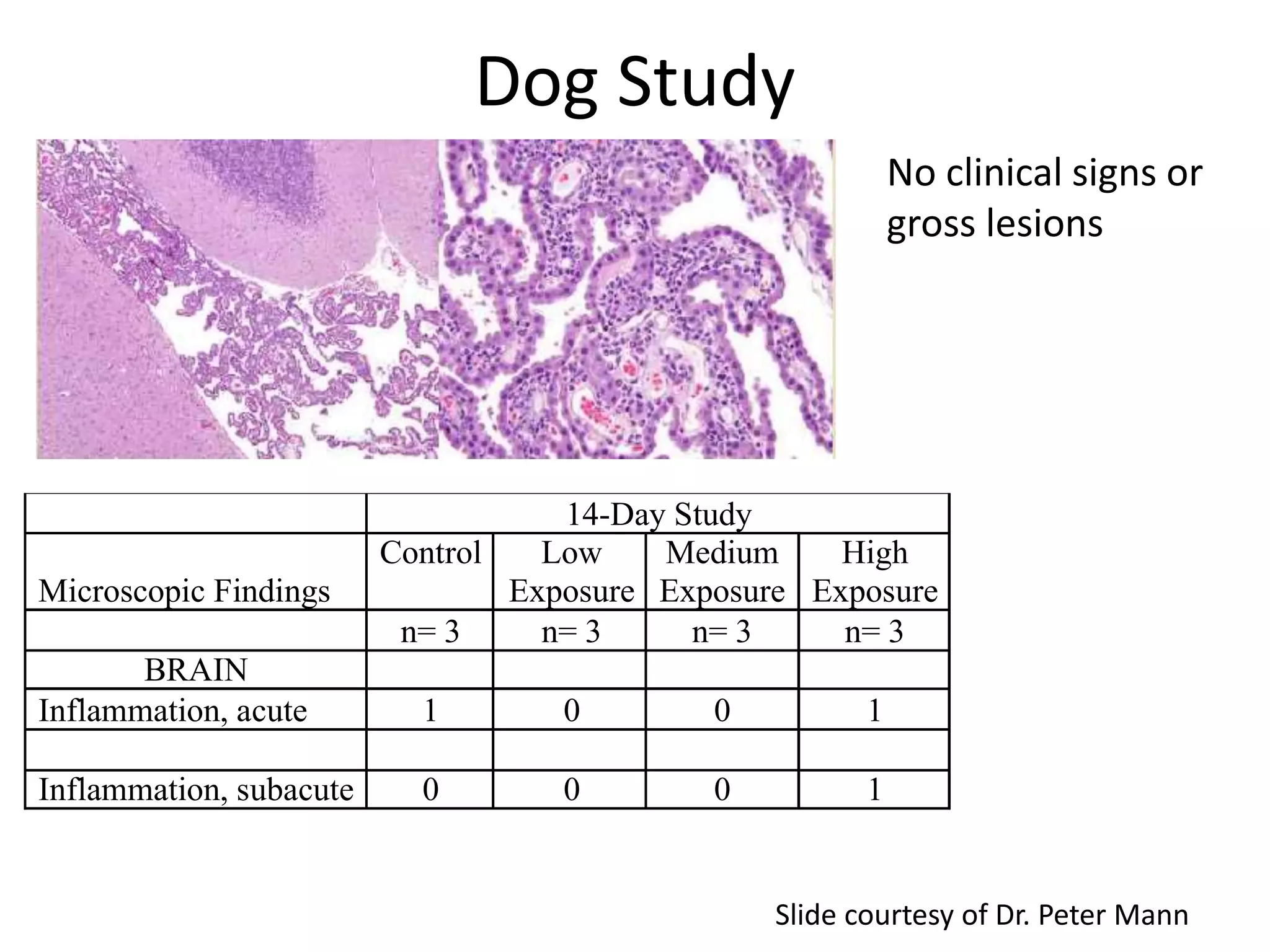
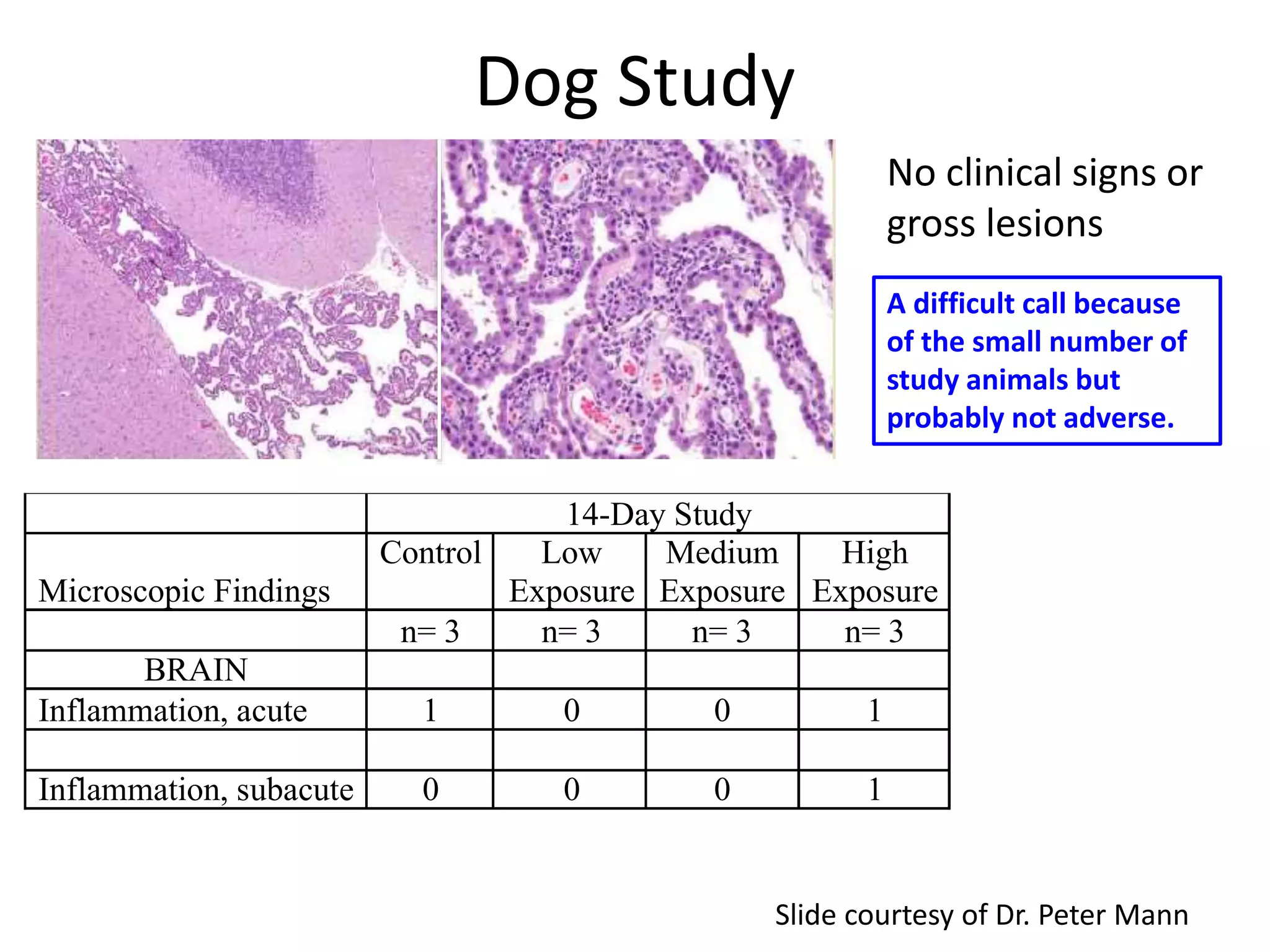
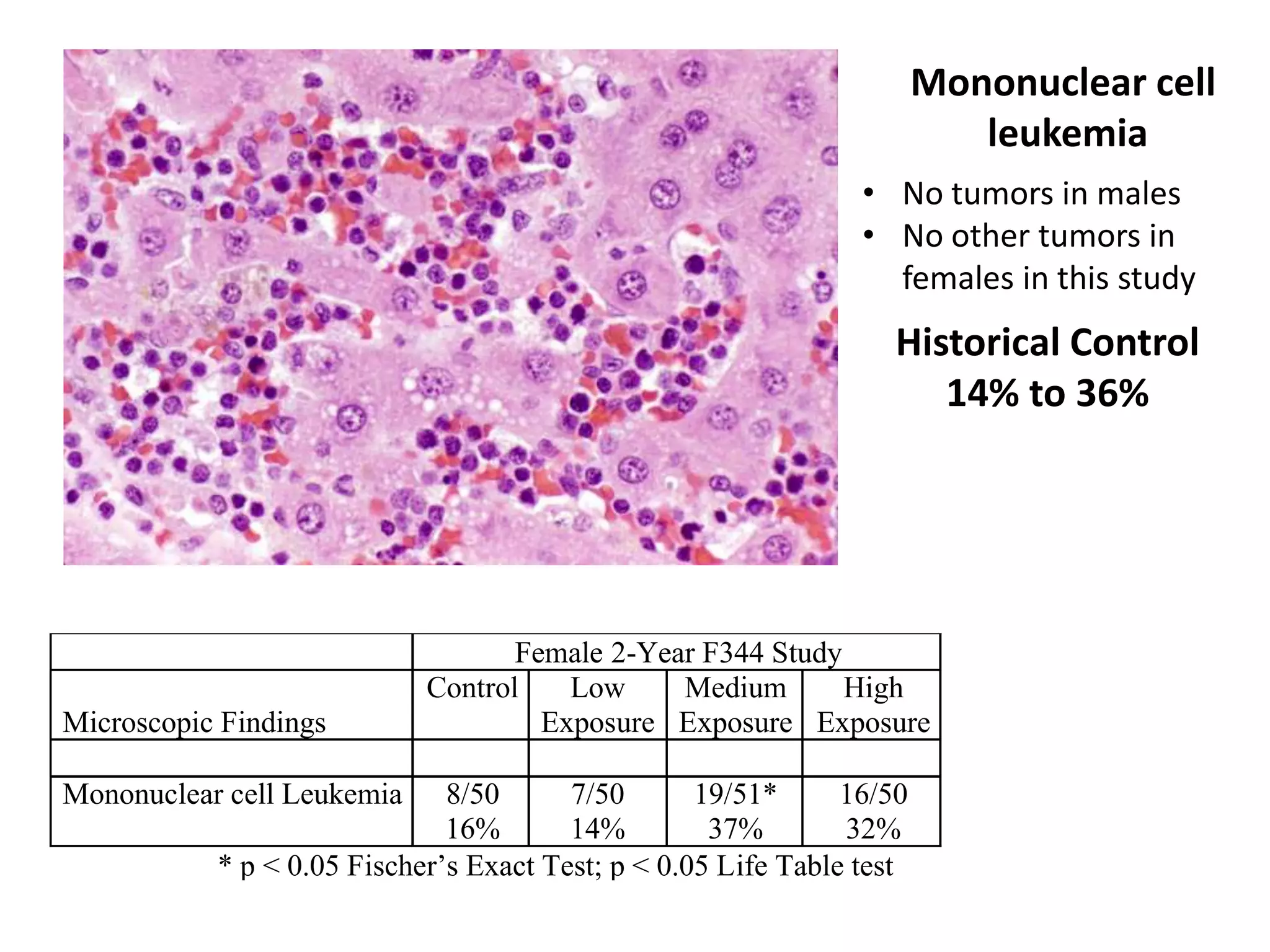
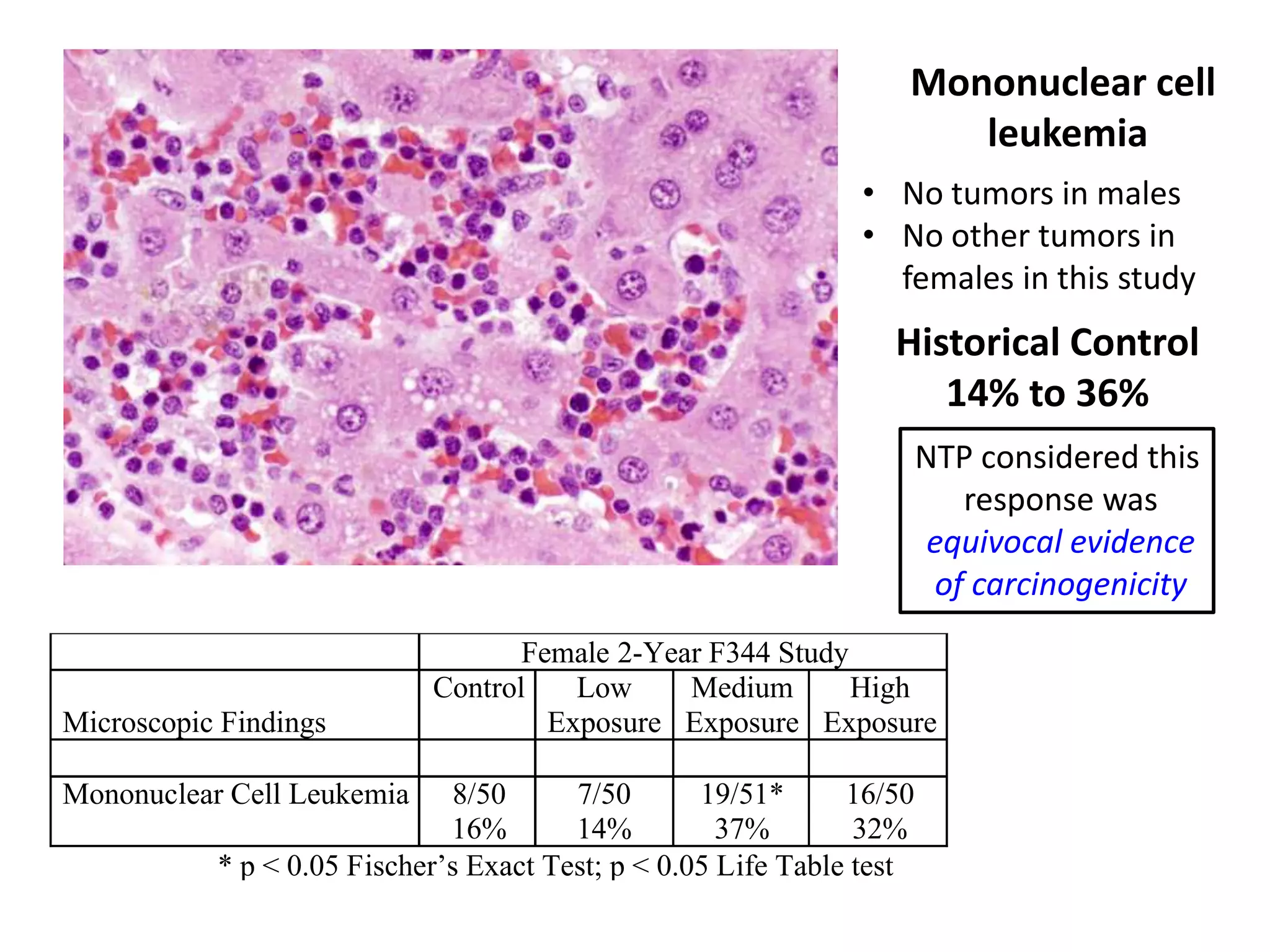
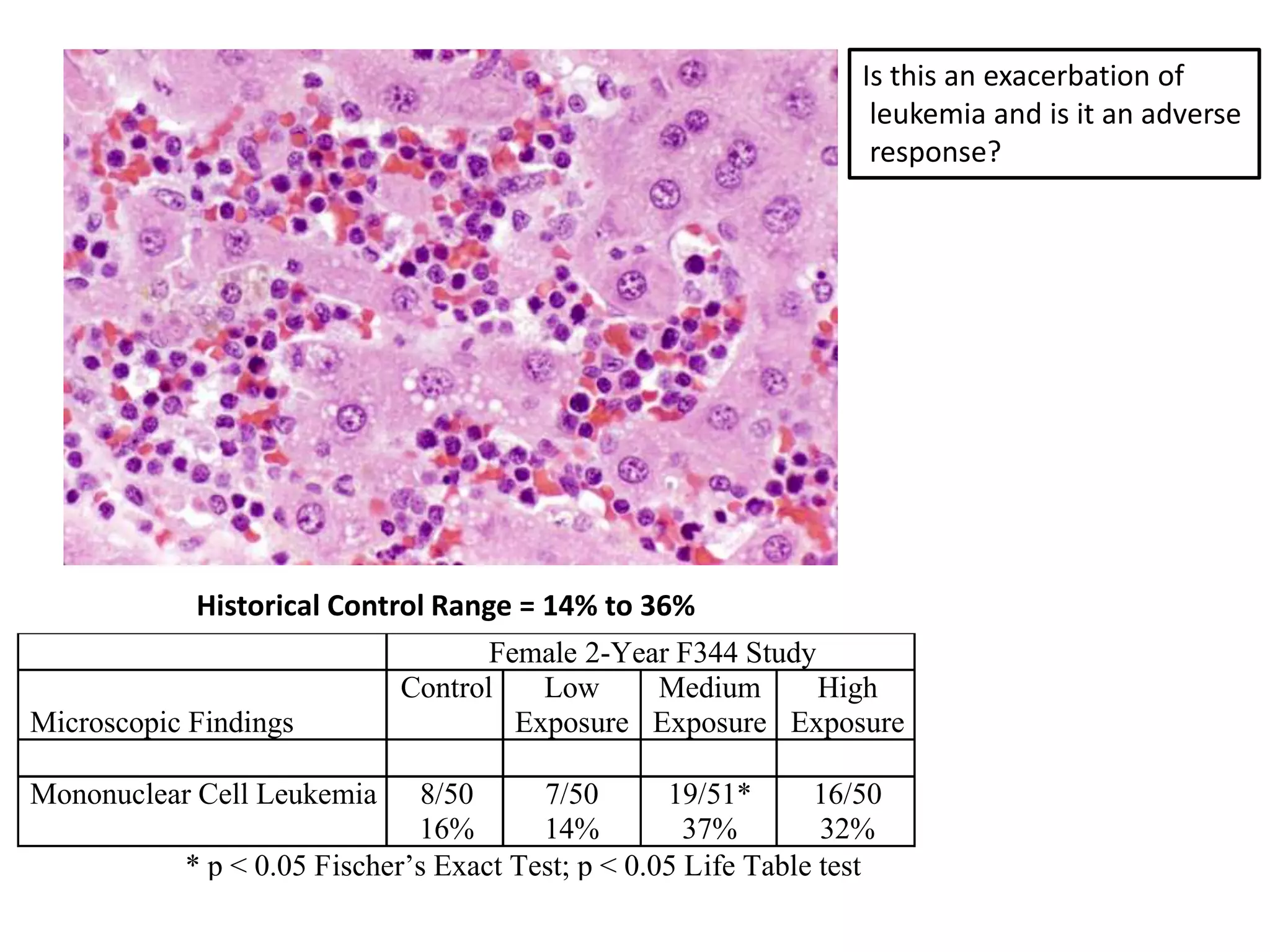
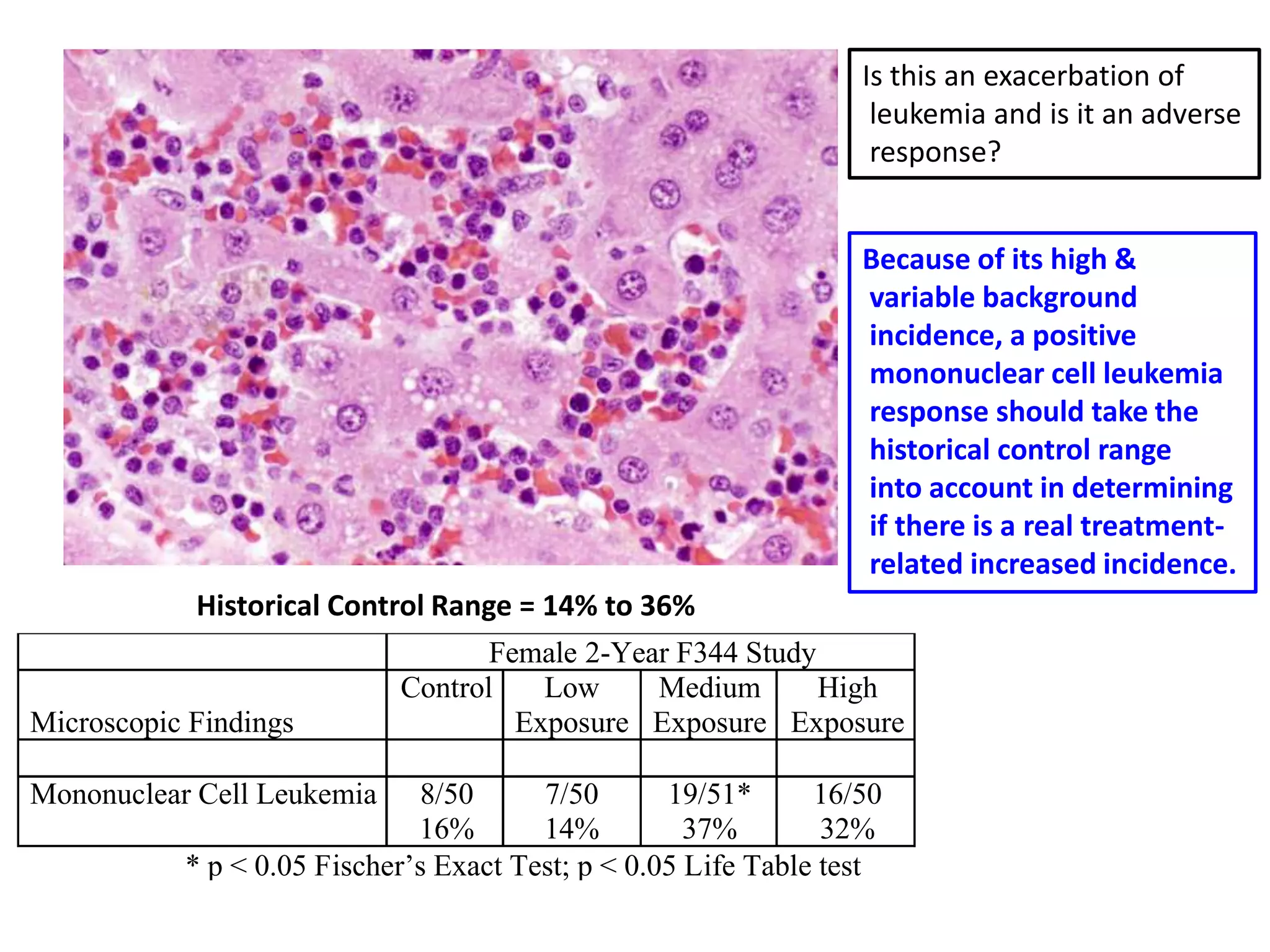
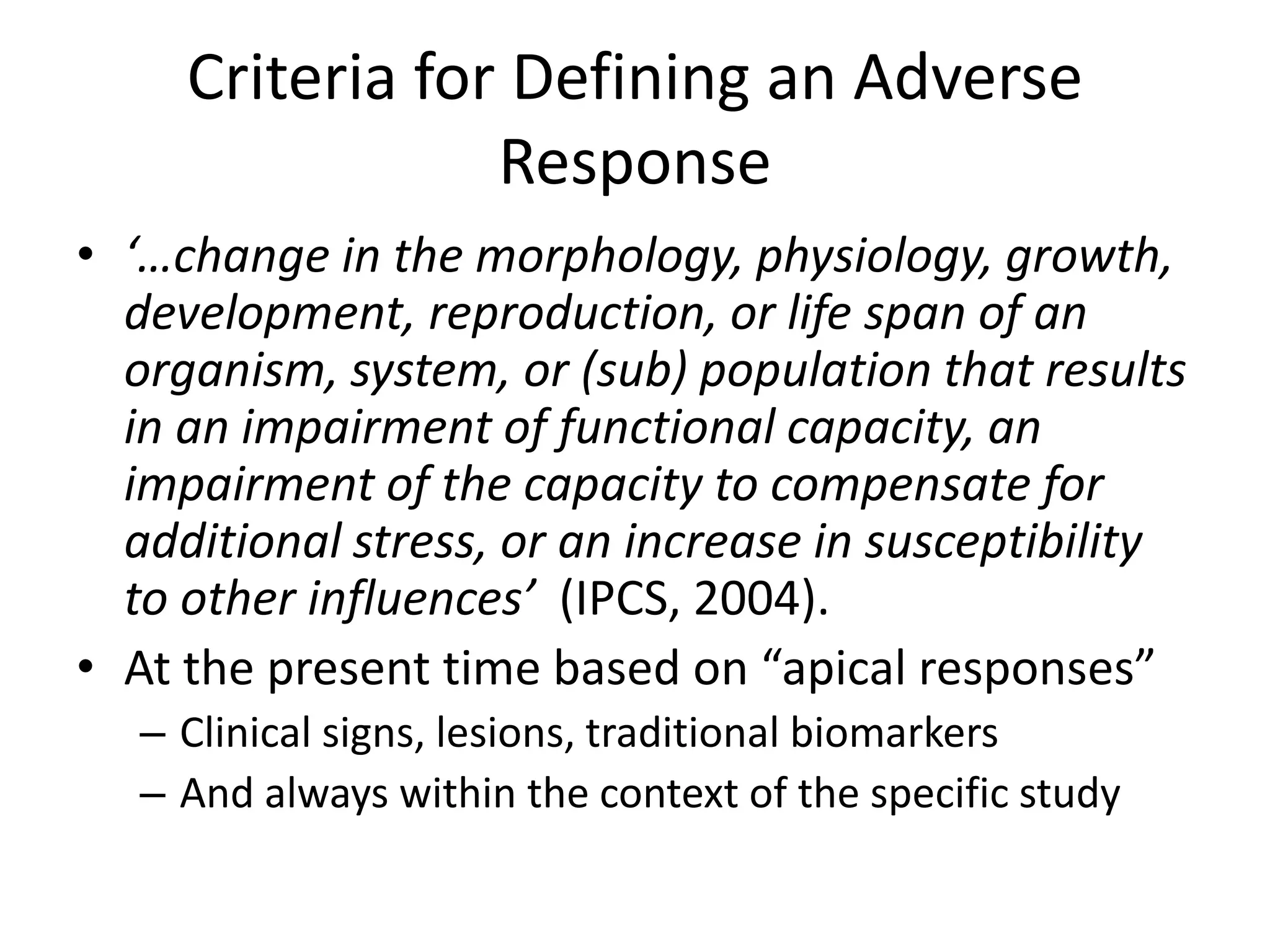
The document discusses the definitions and distinctions between adverse, adaptive, and non-adverse responses in toxicologic pathology, particularly within preclinical studies. It highlights the challenges in defining adverse responses, the criteria for establishing no observed adverse effect levels (NOAEL), and the significance of adaptive responses which may sometimes be adverse. Recommendations for evaluating and reporting toxicological findings are also provided, emphasizing a paradigm shift towards non-animal testing methodologies.