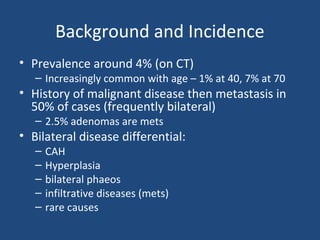
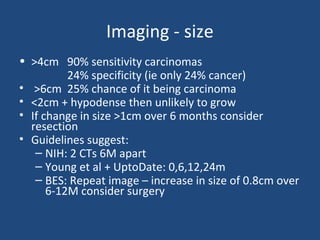
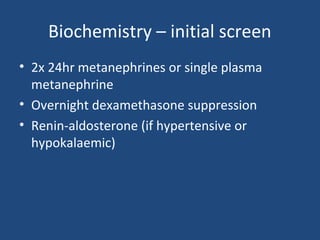
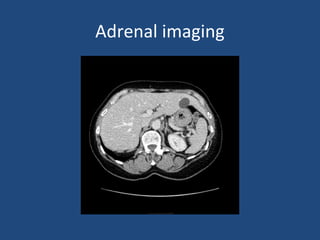
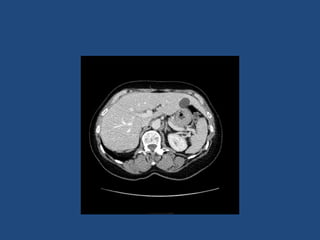
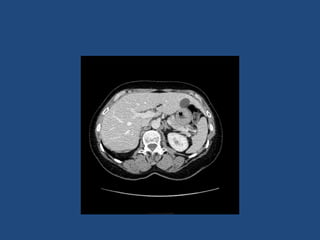
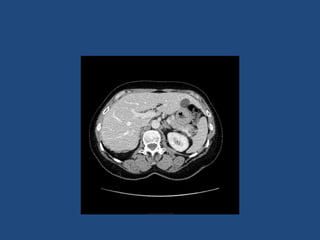
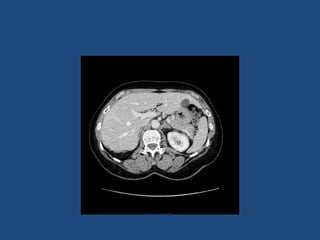
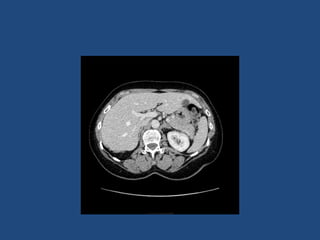
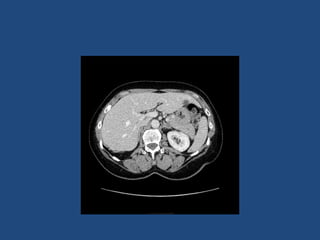
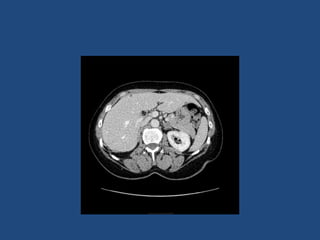
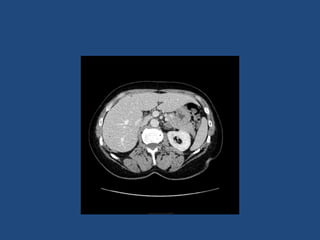
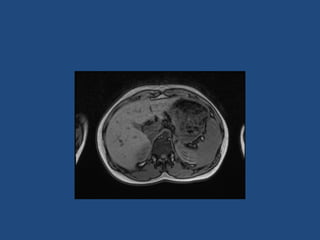
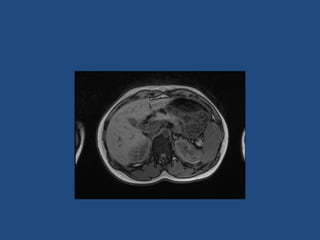
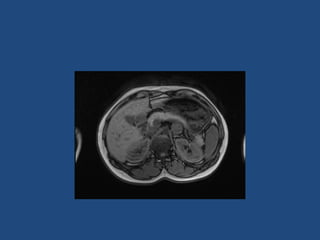
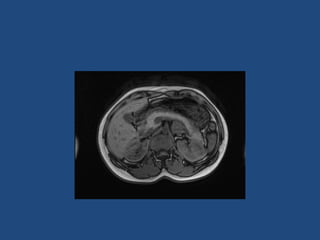
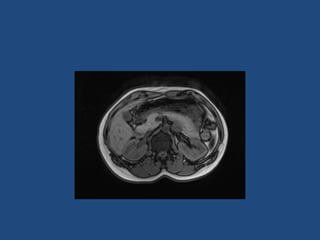
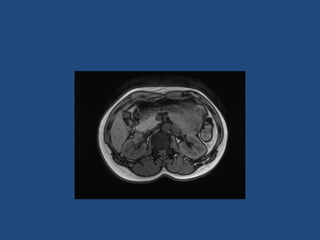
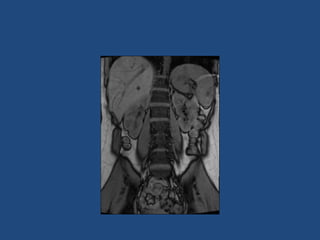
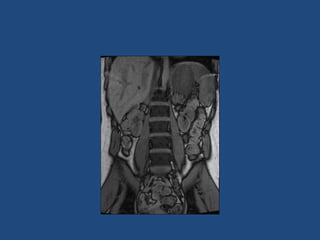
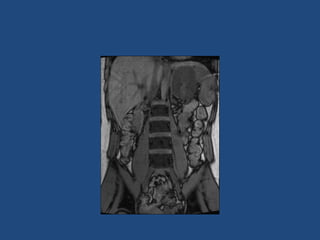
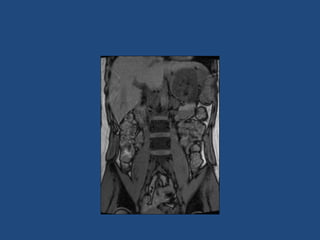
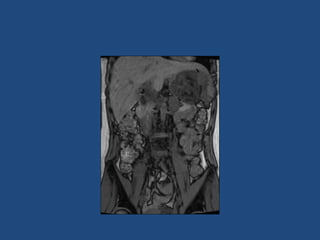
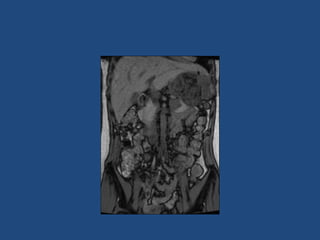
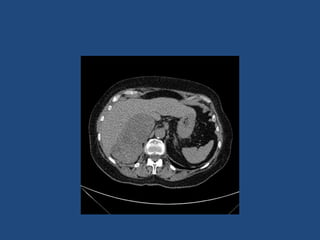
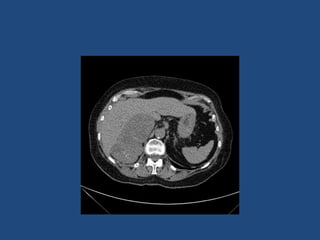
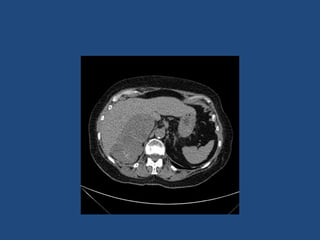
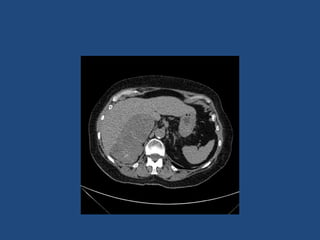
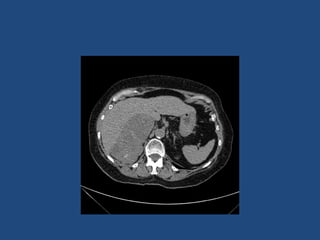
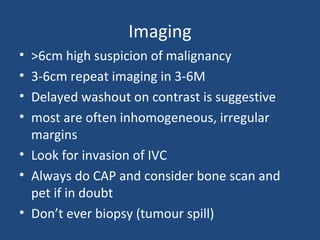
This document discusses adrenal incidentalomas, which are adrenal masses greater than 1cm discovered incidentally on imaging. It covers the epidemiology, risks of progression, imaging techniques, and assessment of hormonal functionality. For hormonally inactive incidentalomas, the risks of malignancy and developing hormonal hypersecretion are low. Dedicated adrenal imaging can help characterize lesions and determine need for follow up. Biochemical testing assesses for hormonal hypersecretion from conditions like pheochromocytoma, Cushing's syndrome, and primary hyperaldosteronism. Subclinical Cushing's syndrome is defined and testing approaches are outlined. Surgical resection may be considered for larger lesions or biochemically active