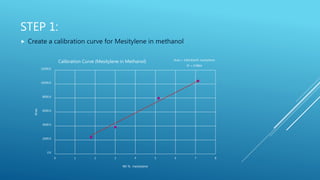
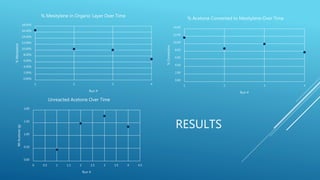
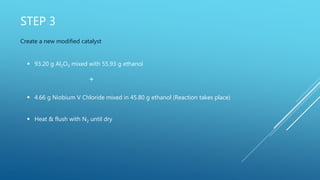
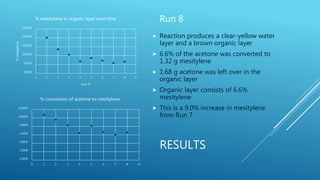
The document describes experiments to develop an efficient catalyst for converting acetone to mesitylene. Initial experiments used an unmodified Al2O3 catalyst, which became less effective over time, producing decreasing yields of mesitylene from 11.6% to 7.8%. A modified catalyst was then created by adding niobium chloride to Al2O3. This catalyst had varying effectiveness over 8 runs, with mesitylene yields ranging from 20.1% to 5.8% and decreasing over time as the catalyst broke down. The goal was to create a catalyst that yields at least 90% mesitylene and breaks down slowly over multiple conversions.