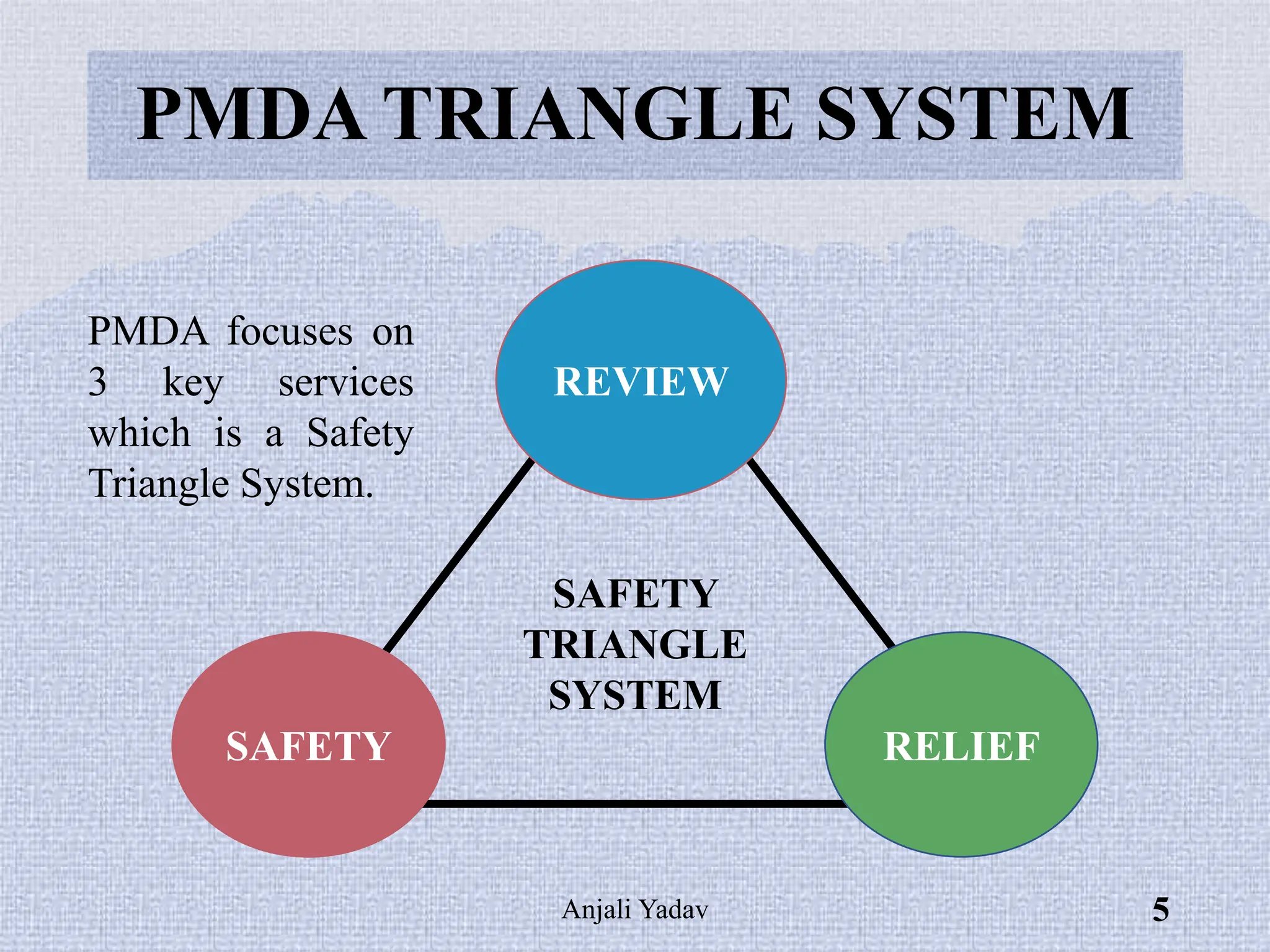
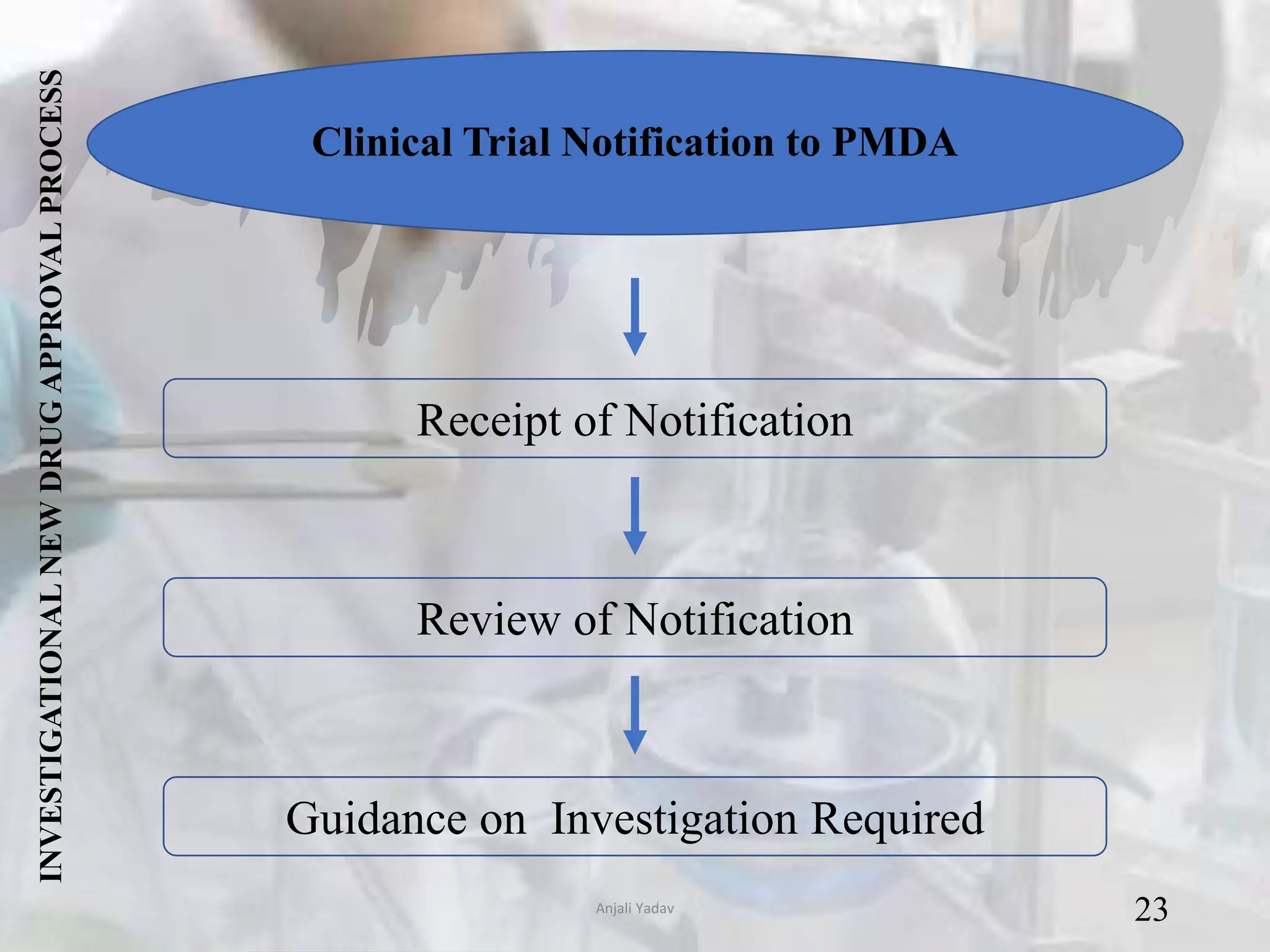
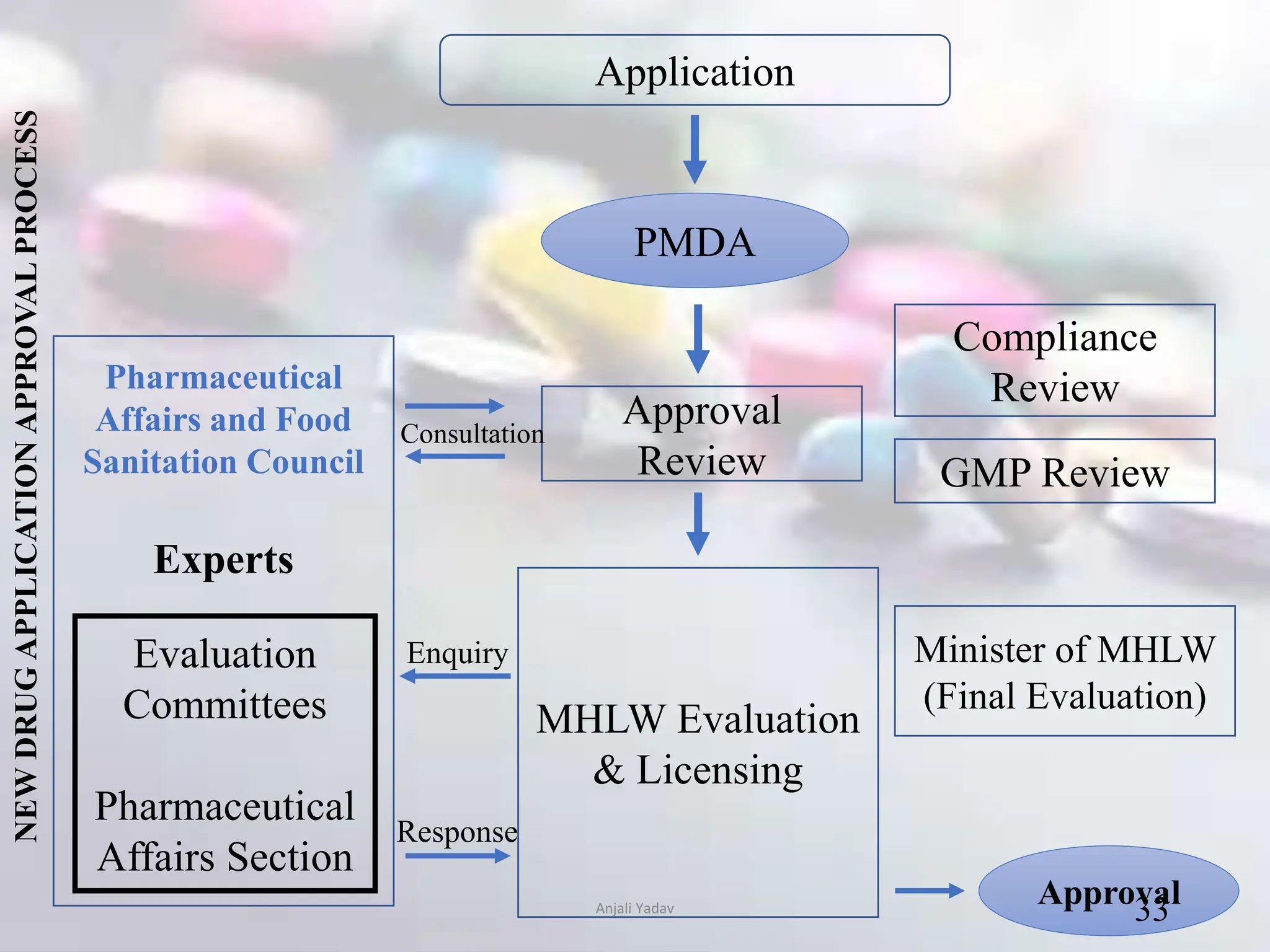
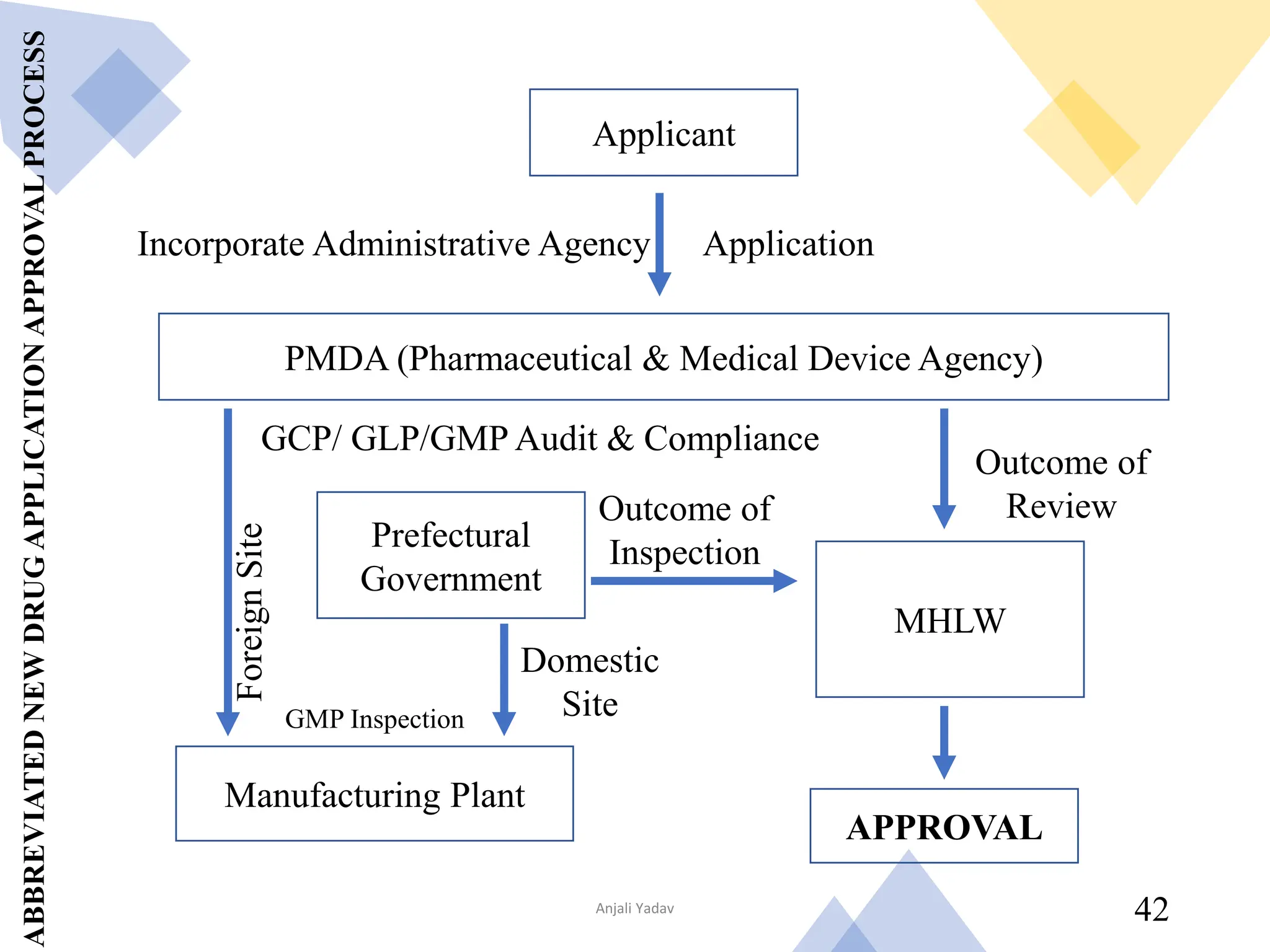
The document presents an overview of the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, highlighting its role in regulating drugs and medical devices alongside the Ministry of Health, Labor, and Welfare. It details various pharmaceutical laws and regulations governing the approval and monitoring of pharmaceuticals, such as the Pharmaceutical and Medical Device Act, and outlines the types of registration applications required for market entry, including those for New Drugs, Abbreviated New Drugs, and Biologics. Additionally, the document emphasizes the importance of safety, efficacy, and quality in the regulatory processes to protect public health.